Left ventricular diastolic dysfunction (LVDD) is an important pathogenic factor for atrial fibrillation (AF). There are few data on the effect of LVDD on recurrence of AF after catheter ablation. A cohort of 124 patients (59.9 ± 11.7 years, 73.9% male, and 55% with paroxysmal AF) with recalcitrant AF and normal left ventricular systolic function (left ventricular ejection fraction ≥50%) undergoing ablation was studied. Each patient underwent transthoracic echocardiography, and LVDD was meticulously graded using rhythm-independent (AF or sinus rhythm) transmitral and tissue Doppler parameters. Patients underwent catheter ablation of AF using a stepwise protocol. All patients were followed up at 3, 6, and 12 months with recurrent AF (>30 seconds) captured by electrocardiography and/or 7-day monitor. Kaplan-Meier survival analysis and Cox proportional hazards model were used. There was no LVDD in 72 patients (58%), whereas 33 (26.6%), 10 (8.1%), and 9 (7.3%) patients had grades 1, 2, and 3 LVDD, respectively. AF recurred in 49 patients (39.5%) with median time to recurrence of 248 days. Patients with higher grade of LVDD were increasingly more likely to have recurrence (37.5% for no LVDD and 30.3%, 60%, and 66.7% for grades 1, 2, and 3 LVDD, respectively). Significant LVDD (grade 2 or 3) was an independent predictor of recurrence (hazard ratio 2.6, p = 0.009) after adjusting for persistent (vs paroxysmal) AF and left atrial volume. In conclusion, patients with more severe LVDD have a higher risk of AF recurrence after catheter ablation. These patients may derive less benefit from ablation or may require a more extensive ablation approach.
Catheter ablation of atrial fibrillation (AF), a common management strategy in symptomatic AF patients resistant to antiarrhythmic medications, is often associated with recurrence of both AF and atrial flutter. Left atrial (LA) remodeling is one of the most important factors to predict the recurrence of AF after catheter ablation. There is paucity of data evaluating the effect of left ventricular diastolic dysfunction (LVDD) on the outcome of catheter ablation for AF. Few studies evaluating the effect of LVDD on the outcome of catheter ablation have been performed and are limited by inadequate, nonuniform, or nondiscriminatory evaluation of LVDD and have failed to discriminate between the effect of structural atrial remodeling and LVDD. Our study systematically evaluates the effect of LVDD on AF recurrence in patients undergoing catheter ablation.
Methods
Consecutive patients who underwent catheter ablation were enrolled in a data registry to evaluate outcomes of ablation. For this study, patients with normal left ventricular (LV) systolic function who had available predictor data and adequate follow-up were included in this retrospective analysis. The institutional review board approved the study, and all the participating patients gave written informed consent for review of data for the purpose of research. Patients were otherwise treated according to standard of care. Patients with ≥2+ mitral regurgitation, mitral stenosis, significant mitral annular calcification, a mitral ring, or a mitral valve prosthesis, which can affect the tissue Doppler evaluation of LVDD, were excluded from the study.
A comprehensive clinical history was obtained and physical examination was performed on all the patients at the time of enrollment. All patients underwent a detailed transthoracic echocardiography and laboratory evaluation at baseline.
All patients underwent evaluation of LV systolic function; LA and LV chamber sizes and volumes; LV wall thickness; and LV diastolic function with mitral inflow Doppler and tissue Doppler. LV volumes were assessed by Simpson’s method with measurements made in 4-chamber and 2-chamber views, and LV ejection fraction was calculated. LV mass was calculated from the LV chamber volumes and LV wall thickness measured at end-diastole. LA chamber dimensions and volumes were measured in 2-chamber and 4-chamber views using Simpson’s and area-length methods. LVDD was assessed and graded according to American Society of Echocardiography recommendations using parameters unaffected by patient’s rhythm (sinus rhythm or AF). LA volumes were not used in grading patient’s LVDD, as they were used as an independent parameter for assessing the risk of recurrent AF. LVDD was graded 0 to 3 as described in the following:
Grade 0: early diastolic velocity of the septal mitral annulus by tissue Doppler (e′) ≥8 cm/s.
Grade 1: e′ <8 cm/s and deceleration time from maximum early ventricular filling velocity to baseline >200 ms.
Grade 2: e′ <8 cm/s and deceleration time 160 to 200 ms.
Grade 3: e′ <8 cm/s and deceleration time <160 ms.
All echocardiographic measurements were assessed by 2 expert echocardiographers (PK and AP) who were blinded to patient outcomes data. The physicians performing catheter ablation were blinded to the LVDD data on the patients.
Catheter ablation was performed on all patients at the University of North Carolina Hospitals’ Electrophysiology Laboratory. Details of the ablation procedure have been previously published. In brief, LA access was achieved by double transseptal puncture. Antral isolation was performed using an irrigated ablation catheter (Chilli II; Boston Scientific, Natick, MA) guided by a circular mapping catheter and impedance-based mapping system (EnSite/NavX; St Jude Medical, St. Paul, MN).
In those with persistent AF, after antral isolation, catheter ablation of AF was performed using a stepwise protocol including complex fractionated atrial electrographic ablation and linear ablation. Linear ablation lesions included a roofline connecting the right and left superior pulmonary veins, a mitral isthmus line connecting the left inferior pulmonary vein to the mitral annulus, and lower posterior LA line to isolate the coronary sinus. If the rhythm organized to an atrial flutter or atrial tachycardia, this was mapped and ablated. Patients underwent cardioversion with ibutilide and/or direct-current cardioversion if they were still in AF after completion of the ablation protocol. Cavotricuspid isthmus ablation was performed in all patients with documented atrial flutter and in all patients with persistent AF. For ablation with closed irrigation catheter, power of 25 to 40 W was used with a target catheter-tip temperature of 41°C. Power was reduced to 25 to 30 W while ablating on the posterior wall, and esophageal temperature monitoring was used to reduce the chance of esophageal injury.
Patients were followed up at 6 weeks and 3, 6, and 12 months after the ablation procedure. At each follow-up, a clinical interview, full clinical examination, and electrocardiography were performed. Patients with implanted pacemaker or implantable cardioverter-defibrillator had interrogation of their device to look for any evidence of AF at each of their visits. Long-term cardiac monitoring was used at 3 and 12 months to assess for asymptomatic AF. Recurrence was defined as >30 seconds of documented AF or atrial flutter and/or atrial tachycardia occurring after a blanking period of 3 months after ablation.
Characteristics of the cohort were analyzed using measures of central tendency. Descriptive characterization of continuous variables was calculated as mean and SD, and frequencies were calculated for categorical variables. Arrhythmia-free survival curves were charted by Kaplan-Meier analysis first for all patients in the cohort. Multivariable Cox proportional hazards models were used including the prespecified covariates: AF pattern (persistent vs paroxysmal) and LA volume. Using the log-rank test, AF recurrence was compared in patients with paroxysmal or persistent AF, with and without diastolic dysfunction. Kaplan-Meier analysis was used to draw arrhythmia-free survival curves in all the patients and in patients with various grades of LVDD. All analyses were performed using statistical software Stata, version 11.0 (StataCorp LP, College Station, Texas).
Results
The study population ( Table 1 ) consisted of 124 patients with AF undergoing a first catheter ablation for AF. Mean age of the cohort was 59.9 ± 11.7 years and 74% were male. AF was persistent in 45% of the patients, and the duration of AF before catheter ablation procedure was 62.8 ± 77.0 months. Risk factors of AF have been summarized in Table 1 . Mean CHADS 2 score was 1.0 in these patients. Almost 60% of the patients were receiving β blockers, and most patients were receiving antiplatelets or anticoagulants ( Table 1 ). All patients had failed a trial of antiarrhythmic drug therapy before ablation. A majority (63%) of the patients were receiving antiarrhythmic medications beyond 3 months after catheter ablation.
| Variables | Overall (N = 124) | Recurrence After Ablation | P ∗ | |
|---|---|---|---|---|
| Yes (N = 49) | No (N = 75) | |||
| Age (years) | 59.9 ± 11.7 | 61.7 ± 10.7 | 58.7 ± 12.3 | 0.41 |
| Men | 74% | 76% | 73% | 0.83 |
| BMI (kg/m 2 ) | 32.0 ± 7.5 | 32.7 ± 8.0 | 31.5 ± 7.1 | 0.50 |
| AF duration (months) | 62.8 ± 77.0 | 63.7 ± 73.6 | 62.2 ± 79.4 | 0.96 |
| Persistent AF | 45% | 59% | 36% | 0.009 |
| Hypertension | 57% | 58% | 57% | 0.92 |
| Diabetes mellitus | 22% | 22% | 22% | 0.94 |
| Coronary artery disease | 18% | 11% | 23% | 0.09 |
| Heart failure | 12% | 11% | 13% | 0.87 |
| Tobacco use | 7.0% | 4.4% | 8.6% | 0.56 |
| Medications | ||||
| Beta-blocker | 60% | 55% | 63% | 0.39 |
| Calcium-channel blocker | 22% | 20% | 23% | 0.88 |
| ACE-inhibitor/angiotensin receptor blocker | 42% | 45% | 40% | 0.66 |
| Statin | 38% | 37% | 39% | 0.31 |
| Aspirin | 58% | 59% | 58% | 0.67 |
| Warfarin | 59% | 61% | 58% | 0.88 |
| Dabigatran | 18% | 18% | 18% | 0.38 |
| CHADS 2 | 1.0 ± 1.0 | 1.0 ± 1.0 | 1.0 ± 1.0 | 0.84 |
| AAD beyond 3 months | 66.9% | 82.6% | 63.4% | 0.02 |
∗ p Value for univariable predictor using Cox proportional hazard analysis.
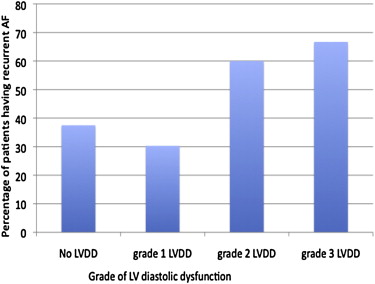
LVDD was present in 52 patients (42%). LVDD was present in 45% of the patients who had recurrent AF and in 40% of the patients who did not have recurrent AF after catheter ablation. Analyzing the patients having recurrent AF by grade of LVDD showed increasing fraction of patients with higher grade, with 37.5% and 30.3%, 60%, and 66.7% of patients with no LVDD and grades 1, 2, and 3 LVDD, respectively having recurrent AF ( Figure 1 ). There was a clear step-up in recurrence rate with more than grade 1 LVDD. LA diameter of the patients was 43 ± 7 mm and LA volume was 80 ± 34 ml. LA diameter and LA volume were not significantly different in patients with and without AF recurrence (LA diameter: 44 ± 7 vs 43 ± 8 mm, p = 0.16; LA volume: 81 ± 32 vs 79 ± 37 ml, p = 0.66). LV systolic function and LV end-diastolic dimension were 62 ± 9% and 49 ± 6 mm, respectively, with no significant difference between patients who had recurrence of AF compared with the patients without recurrence ( Table 2 ).
| Variable | Overall (N = 124) | Recurrence After Ablation | p ∗ | |
|---|---|---|---|---|
| Yes (N = 49) | No (N = 75) | |||
| LV ejection fraction (%) | 62 ± 9 | 60 ± 9 | 63 ± 10 | 0.61 |
| LV end diastolic diameter (mm) | 49 ± 6 | 48 ± 6 | 49 ± 7 | 0.32 |
| LV mass (g) | 204 ± 63 | 204 ± 54 | 204 ± 69 | 0.99 |
| LA diameter (mm) | 43 ± 7 | 44 ± 7 | 43 ± 8 | 0.16 |
| LA area (cm 2 ) | 22 ± 6 | 23 ± 6 | 21 ± 6 | 0.22 |
| LA volume (ml) | 80 ± 34 | 81 ± 32 | 79 ± 37 | 0.66 |
| Diastolic grade | ||||
| 0 | 72 | 27 | 45 | Ref |
| 1 | 33 | 10 | 23 | 0.33 |
| 2 | 10 | 6 | 4 | 0.04 |
| 3 | 9 | 6 | 3 | 0.11 |
∗ p Value for univariable predictor using Cox proportional hazard analysis.
During the mean follow-up of 354 days, recurrent AF was seen in 49 patients (39.5%). Median time to recurrence was 248 days. Univariate Cox proportional hazards analysis demonstrated persistent AF and LVDD grade 2 or 3 as significant predictors of AF recurrence. No other patient characteristics, including age, gender, duration of AF, co-morbidities, medications, or CHADS 2 score, were predictors of AF recurrence on univariate analysis ( Table 1 ).
In multivariate analysis ( Table 3 ) including LVDD, AF pattern, and LA volume, both AF pattern and significant LVDD remained significant determinants of AF recurrence. Patients with persistent AF were >2 times more likely to have AF recurrence (hazard ratio 2.01, 95% confidence interval 1.11 to 3.65, p = 0.022; Figure 1 ). Presence of LVDD grade 2 or 3 increased the risk of recurrence of AF by >2.5 times compared with no LVDD or grade 1 LVDD (hazard ratio 2.58, 95% confidence interval 1.27 to 5.25, p = 0.009; Figure 2 ).



