A randomized, double-blind, placebo-controlled, dose-escalation study was conducted to examine the efficacy and safety of mipomersen (ISIS 301012), an antisense inhibitor of apolipoprotein B, when added to conventional lipid-lowering therapy for patients with heterozygous familial hypercholesterolemia. A total of 44 patients were enrolled and were separated into 4 cohorts, with doses ranging from 50 to 300 mg (4:1 active treatment/placebo ratio). Patients received 8 doses subcutaneously during a 6-week treatment period. Patients assigned to the 300-mg dose continued for an additional 7 weeks with once-per-week dosing. The primary efficacy end point was the percentage of change from baseline to week 7 in low-density lipoprotein (LDL) cholesterol. Safety was assessed using the laboratory test results and according to the incidence, severity, and relation of adverse events to drug dose. Mipomersen produced significant reductions in LDL cholesterol and other atherogenic apolipoprotein B-containing lipoproteins. After 6 weeks of treatment, the LDL cholesterol level was reduced by 21% from baseline in the 200-mg/week dose group (p <0.05) and 34% from baseline in the 300-mg/week dose group (p <0.01), with a concomitant reduction in apolipoprotein B of 23% (p <0.05) and 33% (p <0.01), respectively. Injection site reactions were the most common adverse event. Elevations in liver transaminase levels (≥3 times the upper limit of normal) occurred in 4 (11%) of 36 patients assigned to active treatment; 3 of these patients were in the highest dose group. In conclusion, mipomersen has an incremental LDL cholesterol lowering effect when added to conventional lipid-lowering therapy.
A substantial proportion of patients at high risk of cardiovascular disease remain unable to achieve optimal low-density lipoprotein (LDL) cholesterol concentrations despite the advent of potent statins and the use of combination lipid-lowering therapy. This group has included patients with familial hypercholesterolemia, which is characterized by baseline LDL cholesterol levels readily >500 mg/dl. In these patients a 50% to 60% reduction in LDL cholesterol using statin-ezetimibe combination therapy has still resulted in LDL cholesterol levels >200 mg/dl. Because increasing the statin dose offers limited additional efficacy at the expense of significant increases in side effects, additional LDL cholesterol-lowering therapies acting through compensatory mechanisms, are needed. Mipomersen is a second-generation antisense oligonucleotide designed to inhibit synthesis of the human apolipoprotein B-100 (apoB) by the liver. ApoB is an essential component of LDL cholesterol and all other atherogenic lipoproteins. In previous clinical trials, mipomersen produced dose-dependent and prolonged reductions in LDL cholesterol in healthy volunteers and in patients with mild to moderate hypercholesterolemia. In the present report, we have described the results of a randomized, double-blind, placebo-controlled, dose-escalation, Phase II study designed to evaluate the efficacy and safety of mipomersen when combined with conventional lipid-lowering therapy in patients with familial hypercholesterolemia.
Methods
The present study was designed by the academic investigators in cooperation with the study sponsor, Isis Pharmaceuticals (Carlsbad, California). All data were generated by the academic investigators. The clinical database was maintained by the sponsors. Although we allowed the sponsor to review the report, all data analyses and interpretation of the results were done by the academic investigators. This trial has been registered at Clinicaltrials.gov as NCT00281008 .
The eligible participants were men and women aged 18 to 75 years with heterozygous familial hypercholesterolemia, as defined by a documented history of untreated LDL cholesterol >200 mg/dl (5.2 mmol/L) and the existence of at least one of the following attributes: the presence of a known mutation in the LDL receptor gene; the presence of tendinous or cutaneous xanthomas; an adult first-degree relative with documented LDL cholesterol >190 mg/dl (4.9 mmol/L) or a child <18 years old with LDL cholesterol >130 mg/dl (3.4 mmol/L) before lipid-lowering therapy; or a history of early coronary artery disease in a first-degree relative (male <55 years; female <60 years). The study participants had a fasting LDL cholesterol of ≥130 mg/dl (3.4 mmol/L) and triglycerides <400 mg/dl (4.6 mmol/L) at screening. The patients received stable conventional lipid-lowering therapy for ≥4 weeks and consumed a low-fat diet for ≥8 weeks before the first dose of the study drug and throughout the study. Those subjects with a recent cardiovascular event or related surgical intervention were excluded, as were those with documented history of hepatic, renal, or uncontrolled endocrine disorders. The subjects with a serum creatine phosphokinase level of ≥3 times the upper limit of normal (ULN) or hepatic transaminase levels of ≥2 times the ULN at screening were also excluded from the trial. All participants gave written informed consent before enrollment.
A multicenter, randomized, placebo-controlled, double-blind, dose-escalation design was used for this Phase II study. The eligible participants were randomized 4:1, active treatment to placebo, by dose cohort. The study included 4 dose cohorts of 50, 100, 200, or 300 mg/week of mipomersen (∼10 patients per cohort). The 50- and 100-mg/week cohorts were enrolled first, in parallel, followed by sequential enrollment of the 200- and 300-mg/week cohorts. Enrollment of the next cohort was initiated on demonstration of a satisfactory safety profile after 6 weeks of dosing (week 7) in the preceding cohorts. The design was amended to allow patients in the 300-mg/week dose group to continue weekly dosing for ≤13 weeks. The patients, investigators, and study staff were unaware of the treatment assignments, with the exception of the pharmacist who prepared the study drug. The study drug was administered by subcutaneous injection at a dose of 50, 100, 200, or 300 mg on days 1, 4, 8, and 11, followed by once-weekly injections on days 15, 22, 29, and 36 (6-week treatment period). Patients in the 300-mg/week dose cohort continued weekly dosing on days 43, 50, 57, 64, 71, 78, and 85 (13-week treatment period). The study drug was supplied as a 1-ml solution containing 200 mg of mipomersen or 0.9% sterile saline in a 2-ml glass vial by Isis Pharmaceuticals.
The local institutional review boards approved the study, which was performed in compliance with the standards of Good Clinical Practice (CPMP/ICH/135/95), the Declaration of Helsinki in its revised edition (Washington 2002), and the requirements of the European Clinical Trial Directive 2001/20/EC (amendment regulations SI 2006/2984).
The laboratory evaluations included routine hematology, blood chemistry, and urinalysis. A full physical examination was performed at screening, week 7 (50-, 100-, and 200-mg/week cohorts), and week 15 (300-mg/week cohort). A 12-lead electrocardiogram was recorded at screening, week 7 (all cohorts), and week 15 (300-mg/week cohort). The electrocardiograms were evaluated by an independent cardiologist. The subjects’ vital signs were recorded at each visit during the treatment period and at the post-treatment evaluation (weeks 7 and 15). The patients were followed up for 5 months after their last dose of study drug. During this follow-up period, the patients returned to the study center for clinical evaluation and laboratory tests once per month for the first 3 months. In the absence of abnormalities, the patients were then monitored by telephone interview for the remainder of the follow-up period.
The fasting blood samples were analyzed for lipids and lipoproteins using MedPace (Cincinnati, Ohio). ApoB, apolipoprotein A1, and lipoprotein(a) [Lp(a)] concentrations were determined by rate nephelometry. The total cholesterol and triglyceride (TG) concentrations were measured by standard enzyme-based colorimetric assays. High-density lipoprotein cholesterol was determined using an enzyme-based colorimetric assay after dextran-sulfate precipitation. Very-low-density lipoprotein cholesterol, LDL cholesterol, and non–high-density lipoprotein cholesterol were calculated. The plasma concentrations of mipomersen were analyzed using a validated hybridization-dependent enzyme-linked immunosorbent assay method at PPD Development (Richmond, Virginia). The lower limit of quantitation was 0.23 ng/ml. The pharmacokinetic parameters for mipomersen were calculated using noncompartmental analysis (WinNonlin, version 5.2, Pharsight, Mountain View, California) for each patient’s plasma drug concentration–time profile obtained by serial blood sampling before and after the last dose. The plasma trough concentrations were determined from samples collected approximately 7 days after the previous dose throughout the treatment period. The area under the plasma concentration–time curve was calculated from 0 to 48 hours after the administered dose using the linear trapezoidal rule. Blood samples were also collected during the follow-up period to determine the drug terminal elimination half-life.
The prespecified primary efficacy end point was the percentage of reduction in LDL cholesterol from baseline to day 43 (week 7) per dose group compared to the pooled placebo group. Exploratory analysis of other lipid and lipoprotein parameters was performed on day 43 for each dose group and day 99 (week 15) for the 300-mg/week dose group. The safety end points were evaluated by treatment group and included all adverse and serious adverse events, laboratory test results, vital signs, and electrocardiographic findings. Safety was assessed according to the incidence, severity, and dose relation of the events and by changes in these parameters.
The sample size was determined by a SD of 18 for the percentage of change from baseline for LDL cholesterol, as derived from previous clinical data sets. According to this variance, a sample size of 8, for both the pooled placebo and 300-mg/week dose groups, would provide 80% power to detect a 30% difference in a change from baseline for LDL cholesterol between the 2 treatment groups using a t test with a 0.05 2-sided significance level.
The study end points were analyzed on the intent-to-treat population (n = 44). Missing values were imputed by identifying the assessment closest to 2 weeks after the last dose of the study drug. Descriptive statistics are presented for the lipid parameters by treatment group. Baseline was defined as the average of all screening values combined with the predose day 1 measurement. The percentage of change in each lipid parameter from baseline to day 43 (week 7) for all dose groups was compared to the data from the pooled placebo group using the exact Wilcoxon rank sum test. An analysis of the changes from baseline to day 99 (week 15) for the 300-mg/week dose group was performed using the paired t test or sign test. The software used for the analyses was Statistical Analysis Systems, version 8.2 (SAS Institute, Cary, North Carolina).
Results
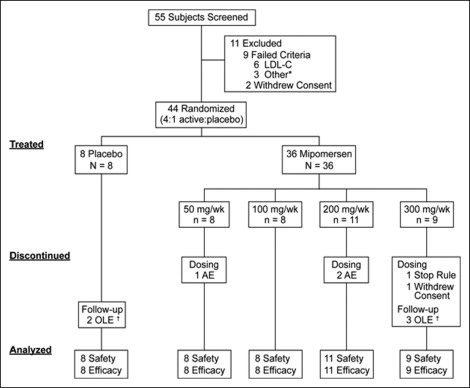
Of the 55 screened patients, 44 were enrolled in the study, at one site in The Netherlands and six sites in the United States, from February 2006 to April 2007 ( Figure 1 ). The demographics and baseline lipid-lowering therapy by treatment group are summarized in Table 1 . Of the 44 patients, 39 completed the treatment period of the study protocol. The 5 patients who did not complete the treatment period were assigned to mipomersen. Of these 5 patients, 4 discontinued dosing because of an adverse event (n = 3) or stopping rule (n = 1). One patient withdrew consent. Also, 5 patients did not enter the follow-up period because of enrollment in an open-label extension study (clinical trial no. NCT00477594 ).
| Placebo (n = 8) | Dose (mg/wk) | |||||
|---|---|---|---|---|---|---|
| 50(n = 8) | 100 (n = 8) | 200 (n = 11) | 300 (n = 9) | Total (n = 44) | ||
| Gender (M:F) | ||||||
| Male | 6 | 5 | 5 | 4 | 6 | 26 |
| Female | 2 | 3 | 3 | 7 | 3 | 18 |
| Age (years) | 54 ± 10 | 49 ± 12 | 53 ± 11 | 56 ± 13 | 47 ± 7 | 52 ± 11 |
| BMI (kg/m 2 ) | 28 ± 3 | 28 ± 4 | 31 ± 5 | 28 ± 8 | 32 ± 6 | 29 ± 6 |
| Lipid-Lowering Medications (n (%)) | ||||||
| Statin | 8 (100) | 6 (75) | 7 (88) | 11 (100) | 9 (100) | 41 (93) |
| Ezetimibe | 6 (75) | 3 (38) | 7 (88) | 7 (64) | 8 (89) | 31 (70) |
| Bile Acid Sequestrant | 2 (25) | 0 (0) | 0 (0) | 3 (27) | 1 (11) | 6 (14) |
| Other | 1 (13) | 0 (0) | 0 (0) | 1 (9) | 2 (22) | 4 (9) |
| Lipid parameters (mg/dl) | ||||||
| LDL cholesterol | 171 ± 47 | 207 ± 76 | 174 ± 40 | 164 ± 30 | 174 ± 35 | 177 ± 47 |
| Very-low-density lipoprotein cholesterol | 35 ± 17 | 25 ± 8 | 27 ± 10 | 28 ± 12 | 34 ± 14 | 30 ± 13 |
| Non–high-density lipoprotein cholesterol | 205 ± 53 | 231 ± 74 | 204 ± 52 | 192 ± 38 | 208 ± 43 | 207 ± 51 |
| High-density lipoprotein cholesterol | 40 ± 6 | 48 ± 10 | 50 ± 15 | 54 ± 16 | 41 ± 7 | 47 ± 12 |
| Total cholesterol | 246 ± 57 | 279 ± 75 | 254 ± 52 | 246 ± 38 | 249 ± 41 | 254 ± 52 |
| Triglycerides | 156 (82–310) | 114 (84–220) | 131 (82–213) | 137 (55–219) | 178 (80–284) | 127 (55–310) |
| Apolipoprotein A1 | 141 ± 23 | 153 ± 26 | 157 ± 31 | 152 ± 29 | 141 ± 18 | 149 ± 26 |
| Apolipoprotein B | 143 ± 31 | 148 ± 37 | 144 ± 38 | 130 ± 26 | 152 ± 24 | 143 ± 31 |
| Lipoprotein (a) | 67 ± 87 | 21 ± 22 | 68 ± 77 | 25 ± 25 | 80 ± 45 | 51 ± 59 |
Statistically significant reductions in apoB and LDL cholesterol were observed in the 200- and 300-mg/week dose groups after 6 weeks of treatment with mipomersen ( Table 2 ). The mean reduction in apoB from baseline to week 7 was 23% in the 200-mg/week dose group and 33% in the 300-mg/week dose group, with parallel reductions in LDL cholesterol of 21% and 34%, respectively. In addition, reductions in Lp(a) and TGs were demonstrated in the 200- and 300-mg/week dose groups; however, these reductions were not statistically significant. Extended treatment to 13 weeks with weekly doses of 300 mg mipomersen resulted in significant mean reductions from baseline to week 15 of 37% in apoB and 37% in LDL cholesterol ( Table 3 ). Lp(a) was lowered by 29%. The LDL cholesterol and apoB levels remained below baseline for ≥3 months after the last dose in the 4 patients who completed both the treatment and the follow-up periods of the study.
| Lipid Parameter | Placebo (n = 8) | Dose (mg/wk) | |||
|---|---|---|---|---|---|
| 50 (n = 8) | 100 (n = 8) | 200 (n = 11) | 300 (n = 9) | ||
| Low-density lipoprotein cholesterol | 0 ± 23 | −13 ± 15 | −11 ± 10 | −21 ± 23 ⁎ | −34 ± 18 † |
| Very-low-density lipoprotein cholesterol | −7 ± 18 | −5 ± 29 | 21 ± 42 | −14 ± 28 | −6 ± 61 |
| Non–high-density lipoprotein cholesterol | −1 ± 22 | −12 ± 16 | −8 ± 11 | −21 ± 19 ⁎ | −31 ± 20 ⁎ |
| High-density lipoprotein cholesterol | 8 ± 17 | −1 ± 13 | −3 ± 20 | −1 ± 13 | 6 ± 11 |
| Total cholesterol | −0 ± 17 | −10 ± 14 | −7 ± 11 | −16 ± 15 ⁎ | −25 ± 17 ⁎ |
| Triglycerides | −16 (−27, 25) | 6 (−60, 25) | 6 (−25, 90) | −23 (−48, 48) | −22 (−62, 137) |
| Apolipoprotein A1 | −0 ± 8 | −3 ± 5 | −4 ± 16 | −2 ± 9 | −2 ± 11 |
| Apolipoprotein B | −1 ± 17 | −10 ± 12 | −8 ± 11 | −23 ± 19 ⁎ | −33 ± 22 † |
| Lipoprotein (a) | −3 ± 21 | −3 ± 10 | −15 ± 10 | −17 ± 19 | −24 ± 26 |
| Lipid Parameter (mg/dl) | Baseline (n = 9) | Day 99 (n = 9) | Change | |
|---|---|---|---|---|
| Absolute | % | |||
| Low-density lipoprotein cholesterol | 174 ± 35 | 109 ± 40 | −66 ± 41 † | −37 ± 21 ‡ |
| Very-low-density lipoprotein cholesterol | 34 ± 14 | 23 ± 7 | −11 ± 15 | −22 ± 37 |
| Non–high-density lipoprotein cholesterol | 208 ± 43 | 131 ± 38 | −77 ± 53 † | −35 ± 20 ‡ |
| High-density lipoprotein cholesterol | 41 ± 7 | 43 ± 9 | 2 ± 7 | 4 ± 16 |
| Total cholesterol | 249 ± 41 | 174 ± 42 | −75 ± 53 † | −29 ± 18 † |
| Triglycerides | 178 (80–284) | 111 (75–193) | −70 (−173, 41) | −38 (−61, 49) |
| Apolipoprotein A1 | 141 ± 18 | 144 ± 20 | 2 ± 21 | 2 ± 16 |
| Apolipoprotein B | 152 ± 24 | 95 ± 35 | −57 ± 38 † | −37 ± 22 † |
| Lipoprotein (a) | 80 ± 45 | 63 ± 56 | −17 ± 24 | −29 ± 30 ⁎ |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


