Adjunctive thrombus aspiration (TA) during primary percutaneous coronary intervention improves myocardial perfusion and survival; however, the effect of effective thrombus retrieval remains unclear. We evaluated whether macroscopic-positive TA in patients with ST-segment elevation myocardial infarction would reduce the infarct size (IS) and microvascular obstruction (MVO), as assessed by contrast-enhanced magnetic resonance imaging. A total of 88 patients with ST-segment elevation myocardial infarction were prospectively recruited and assigned to the TA-positive group (n = 38) or TA-negative group (n = 50) according to whether macroscopic aspirate thrombus was visible to the naked eye. The primary end points were the extent of early and late MVO as assessed by contrast-enhanced magnetic resonance imaging performed during in-hospital stay and IS evaluated in the acute phase and at 6 months of follow-up. The incidence of early and late MVO and IS in the acute phase was lower in the TA-positive group than in the TA-negative group (early MVO 3.8 ± 1.1% vs 7.6 ± 2.1%, respectively, p = 0.003; late MVO 2.1 ± 0.9% vs 5.4 ± 2.9%, p = 0.006; and IS 14.9 ± 8.7% vs 28.2 ± 15.8%, p = 0.004). At the 6-month contrast-enhanced magnetic resonance imaging study, the final IS was significantly lower in the TA-positive group (12.0 ± 8.3% vs 22.3 ± 14.3%, respectively) than in the TA-negative group (p = 0.002). After multivariate adjustment, macroscopic-positive TA represented an independent predictor of final IS (odds ratio 0.34, 95% confidence interval 0.03 to 0.71, p = 0.01). In conclusion, effective macroscopic thrombus retrieval before stenting during percutaneous coronary intervention for ST-segment elevation myocardial infarction is associated with an improvement in myocardial reperfusion, as documented by a clear reduction in the MVO extent and IS.
Primary percutaneous coronary intervention has emerged as the preferred treatment of acute ST-segment elevation myocardial infarction (STEMI). However, despite adequate epicardial reperfusion in the infarct-related artery, spontaneous or primary percutaneous coronary intervention-induced embolization of atherothrombotic material from the culprit lesion into the distal vasculature can occur and can induce persistent impairment of microvascular blood flow in a significant proportion of patients. Microvascular dysfunction has been reported to be associated with larger infarct size (IS), reduced recovery of ventricular function, and increased mortality. To date, scientific evidence has been presented to suggest that adjunctive manual thrombus aspiration (TA) during primary percutaneous coronary intervention improves myocardial reperfusion and decreases mortality in patients with STEMI, potentially by reducing microvascular damage. In the Thrombus Aspiration during Percutaneous coronary intervention in Acute myocardial infarction Study (TAPAS), the largest randomized study of a thrombectomy device, macroscopic aspirate thrombotic material was observed in >½ of the cases. However, the effect of effective thrombus retrieval on myocardial reperfusion remains poorly described. Therefore, we performed a prospective study to evaluate whether positive macroscopic TA in patients with STEMI would reduce the IS and microvascular obstruction (MVO), as assessed by contrast-enhanced magnetic resonance imaging (CE-MRI).
Methods
Patients aged <75 years who had undergone primary percutaneous coronary intervention with manual TA for a first STEMI were recruited. The inclusion criteria were as follows: a diagnosis of STEMI with evidence of ischemic chest pain for >30 minutes and new ST-segment elevation of ≥2 mm in ≥2 contiguous electrocardiographic leads within 12 hours of symptom onset, infarct-related artery ≥2.5 mm in diameter, and angiographically identifiable thrombus with a thrombus score of ≥3. The exclusion criteria were a history of cardiac disease, technical failure of nontraumatic passage of an aspiration catheter owing to severe tortuosity and/or calcification, left main disease, cardiogenic shock, thrombolytic therapy before percutaneous coronary intervention, and a contraindication to CE-MRI. We also excluded patients with diabetes mellitus to limit microvascular dysfunction related to glycemic dysregulation. The local ethics committee approved the study protocol, and all enrolled patients provided informed consent.
All interventions were performed according to the current guidelines. TA was performed with ≥2 passages across the lesion with a 6F Export Aspiration Catheter (Medtronic Vascular, Santa Rosa, California) before balloon dilation and/or stent implantation. The patients were divided into 2 groups. TA was considered effective if macroscopic aspirate thrombus was visible to the naked eye in the dedicated filter basket, and patients were assigned to the TA-positive group. However, if the TA procedure did not retrieve visible atherothrombotic material, the patients were assigned to the TA-negative group. The pre- and postprimary percutaneous coronary intervention Thrombolysis In Myocardial Infarction flow, myocardial blush grade, and thrombus score were estimated visually by 1 experienced observer (R.C.), as previously described. All patients underwent 12-lead electrocardiography at baseline and 90 minutes after revascularization. ST-segment resolution was also measured. Standard therapy was prescribed after primary percutaneous coronary intervention according to current guidelines.
All CE-MRI studies were conducted at 3.0 field strength (Signa HD, General Electric Healthcare, Milwaukee, Wisconsin) and performed in the acute phase and repeated at 6 months. Left ventricular function was assessed by electrocardiographic-gated cine steady-state free precession breath-hold sequences in the 2-chamber and 4-chamber views and the short cardiac axis from the base to the apex (30 phases/cardiac cycle, repetition time 3.5 ms, echo time 1.2 ms, flip angle 45°, typical voxel size 1.92 × 1.25 × 8.0 mm).
CE-MRI was performed at 3 and 15 minutes with a breath-hold electrocardiographic-gated T 1 -weighted sequence after the injection of a bolus of gadolinium (Dota-Gd, Guerbet, Roissy, France) at a single dose of 0.1 mmol/kg (Echo time = minimum full, field of view = 440 mm, inversion time = optimized to obtain an optimal myocardial nulling, matrix 256 × 224, interpolated 256 × 256, slice thickness = 8 mm, gap, 1 mm). The number and position of slices were the same as used for functional imaging.
Image analysis was performed in a blinded fashion by 2 operators (R.C., P.P.) using an off-line dedicated workstation (General Electric Healthcare, Milwaukee, Wisconsin). The left ventricular ejection fraction, end-diastolic and end-systolic volumes, and mass were calculated from the steady-state free precession short-axis views. The left ventricular volume changes were assessed as the percentage of increase or decrease in the left ventricular end-diastolic volume from the baseline to 6-month follow-up examination in each patient (percentage of change in left ventricular end-diastolic volume). Left ventricular remodeling was defined as an increase in the percentage of change in the end-diastolic volume >20%. Early and late MVO was assessed in the initial CE-MRI study performed during the in-hospital stay. IS was assessed from the same initial CE-MRI and at 6 months of follow-up. IS and MVO (if present) were manually traced from the CE-MRI short-axis images. Myocardial regions were considered infarcted if the IS signal intensity was >2 SDs above the remote myocardium. The MVO was defined as a dark zone within the infarcted segments, usually located in the subendocardium ( Figure 1 ). Early MVO was estimated in the sequences at 3 minutes, as previously described, and late MVO at 15 minutes. MVO and IS are expressed in grams (assuming 1.05 g/ml as the specific gravity of the myocardium) and as a percentage of the left ventricular mass.
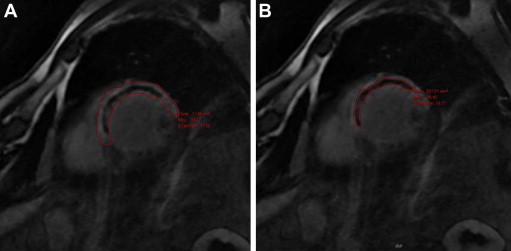
The primary end points were the relation between effective TA and the presence and extent of early and late MVO at the acute phase and IS as evaluated at the acute phase and at 6 months. The secondary end points were the association between positive TA and myocardial blush grade, 90-minute ST-segment resolution, and left ventricular remodeling.
Quantitative variables are presented as the mean ± SD and were analyzed using a 2-tailed Student’s t test or Mann-Whitney U test for non-normally distributed variables. Categorical variables are expressed as the number and percentage and were compared using the chi-square test or Fisher’s exact test. The sample size was calculated as follows: on the basis of an expected absolute difference between groups of 5% in the final IS, with a SD of 3%, at an α risk of 5% and a β risk of 10%, 38 patients were needed to be enrolled in each group. Univariate and multivariate logistic regression analyses were used to identify the predictors of effective TA and final IS at 6 months. The variables that were significantly related to the primary end point on univariate analysis (p <0.1) were included in the multivariate model, and the results are expressed as odds ratios (ORs), with 95% confidence intervals (CIs). A p value <0.05 was considered statistically significant. Statistical analyses were performed with SAS, version 9.2 (SAS Institute, Cary, North Carolina).
Results
From January 2010 to July 2011, 256 patients were admitted to our department because of STEMI. Of these 256 patients, 90 met the inclusion criteria and were screened for the present study. Two patients refused the initial CE-MRI because of severe claustrophobia and were thus excluded. The remaining 88 patients were included in the present study: 38 (43%) in the TA-positive group and 50 (57%) in the TA-negative group.
The baseline clinical and preprocedural angiographic characteristics of the study population are listed in Table 1 . The 2 groups were comparable in age, gender, and risk factors of coronary disease. Six patients (7%) had diabetes mellitus that was diagnosed after in-hospital admission. No significant difference was seen in the delay between the onset of symptoms and urgent revascularization.
| Variable | Total (n = 88) | TA | p Value | |
|---|---|---|---|---|
| No (n = 50) | Yes (n = 38) | |||
| Age (yrs) | 55 ± 10 | 56 ± 11 | 54 ± 10 | 0.459 |
| Men | 73 (83%) | 45 (90%) | 28 (74%) | 0.098 |
| Hypertension | 29 (33%) | 16 (32%) | 13 (34%) | 0.176 |
| Diabetes mellitus | 6 (7%) | 3 (6%) | 3 (8%) | 0.305 |
| Smokers | 50 (56%) | 34 (68%) | 26 (68%) | 0.182 |
| Obesity ∗ | 8 (9%) | 2 (4%) | 6 (15%) | 0.071 |
| Dyslipidemia † | 31 (35%) | 18 (36%) | 13 (34%) | 0.175 |
| Total ischemic time (min) | 349 ± 270 | 325 ± 227 | 412 ± 364 | 0.216 |
| Systolic blood pressure (mm Hg) | 122 ± 23 | 121 ± 23 | 124 ± 23 | 0.524 |
| Diastolic blood pressure (mm Hg) | 77 ± 14 | 75 ± 14 | 79 ± 14 | 0.131 |
| Diseased coronary arteries (n) | ||||
| 1 | 58 (66%) | 31 (62%) | 27 (71%) | 0.496 |
| 2 | 23 (26%) | 14 (28%) | 9 (23%) | 0.807 |
| 3 | 7 (8%) | 5 (10%) | 2 (5%) | 0.457 |
| Infarct-related coronary artery | ||||
| Left anterior descending artery | 30 (34%) | 17 (34%) | 13 (34%) | 0.179 |
| Left circumflex artery | 13 (15%) | 11 (22%) | 2 (5%) | 0.035 |
| Right | 34 (39%) | 13 (26%) | 21 (55%) | 0.007 |
| Other | 11 (12%) | 9 (18%) | 2 (5%) | 0.055 |
| Preprocedural Thrombolysis In Myocardial Infarction flow grade | 0.110 | |||
| 0 or 1 | 59 (67%) | 29 (58%) | 30 (79%) | 0.062 |
| 2 | 7 (8%) | 3 (6%) | 4 (10%) | 0.227 |
| 3 | 22 (25%) | 18 (36%) | 4 (10%) | 0.004 |
| Bifurcation | 16 (18%) | 7 (14%) | 9 (24%) | 0.112 |
| Lesion length (mm) | 24 ± 11 | 12 ± 5 | 12 ± 6 | 0.610 |
| Vessel reference diameter (mm) | 3.1 ± 0.4 | 2.9 ± 0.4 | 3.1 ± 0.3 | 0.003 |
| Preprocedural minimum lumen diameter (mm) | 0.26 ± 0.4 | 0.37 ± 0.4 | 0.19 ± 0.4 | 0.060 |
| Preprocedural thrombus score | ||||
| 3 | 29 (33%) | 17 (34%) | 12 (31%) | 0.068 |
| 4 | 28 (32%) | 15 (30%) | 13 (34%) | 0.457 |
| 5 | 31 (35%) | 18 (36%) | 13 (34%) | 0.259 |
∗ Defined as body mass index ≥30 kg/m 2 .
The infarct-related artery was the right coronary artery in 39%, left anterior descending artery in 34%, and left circumflex artery in 15%. The right coronary artery was significantly more often responsible for infarction in the TA-positive group, and the left circumflex artery was significantly less often the infarct-related artery in the same group. The vessel diameter was different between the 2 groups and was significantly greater in the TA-positive group.
The postprocedural angiographic results are listed in Table 2 . The duration of revascularization did not differ significantly between the 2 groups. The post-stenting minimum lumen diameter was wider in the TA-positive group compared with the TA-negative group (p = 0.003). The rate of angiographic procedural success assessed by the postprocedural Thrombolysis In Myocardial Infarction flow of 3 and myocardial blush grade of ≥2 was 94.3% and 94.6%, respectively, and did not differ between the 2 groups. Similarly, the 90-minute ST-segment resolution, peak cardiac troponin level, administration of glycoprotein IIb/IIIa inhibitors or bivalirudine, and the use of direct stenting did not differ significantly between the 2 groups. The rate of intraprocedural complications was also similar.
| Variable | Total (n = 88) | TA | p Value | |
|---|---|---|---|---|
| No (n = 50) | Yes (n = 38) | |||
| Duration of fluoroscopy (min) | 13 ± 1 | 11 ± 7 | 17 ± 19 | 0.069 |
| Administration of glycoprotein IIb/IIIa inhibitor | 53 (60%) | 32 (64%) | 19 (55%) | 0.359 |
| Anticoagulant therapy (%) | ||||
| Unfractionated heparin | 58 (72%) | 29 (58%) | 19 (50%) | 0.756 |
| Low-molecular-weight heparin | 13 (14%) | 6 (12%) | 7 (18%) | 0.458 |
| Bivalirudin | 17 (19%) | 10 (12%) | 7 (29%) | 0.058 |
| “Direct” stenting | 54 (61%) | 29 (59%) | 25 (65%) | 0.512 |
| Stent type | ||||
| Bare metal stent | 67 (76%) | 36 (72%) | 31 (82%) | 0.325 |
| Drug-eluting stent | 8 (9%) | 4 (8%) | 4 (10%) | 0.721 |
| Minimum lumen diameter after stenting (mm) | 2.9 ± 0.4 | 2.8 ± 0.4 | 3.0 ± 0.4 | 0.003 |
| Postprocedural Thrombolysis In Myocardial Infarction flow grade | ||||
| 0 or 1 | 2 (2%) | 0 (0%) | 2 (5%) | 0.183 |
| 2 | 3 (3%) | 2 (4%) | 1 (2%) | 0.424 |
| 3 | 83 (93%) | 48 (96%) | 35 (92%) | 0.648 |
| Post-stenting myocardial blush grade | ||||
| 0 or 1 | 6 (7%) | 2 (4%) | 4 (13%) | 0.391 |
| ≥2 | 82 (95%) | 48 (96%) | 34 (89%) | 0.721 |
| Intraprocedural complications | ||||
| Side branch occlusion | 3 (3%) | 3 (6%) | 0 (0%) | 0.255 |
| Flow-limiting dissection | 2 (2%) | 1 (2%) | 1 (3%) | 1.000 |
| Distal embolization | 6 (7%) | 4 (8%) | 2 (5%) | 0.694 |
| No-reflow | 2 (2%) | 0 (0%) | 2 (5%) | 0.183 |
| Emergency coronary artery bypass grafting | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 |
| ≥1 Previous criteria | 12 (14%) | 7 (14%) | 5 (13%) | 0.856 |
| 90-minute ST-segment resolution >70% | 74 (84%) | 40 (80%) | 34 (89%) | 0.257 |
| Cardiac troponin peak (ng/ml) | 99 ± 82 | 92 ± 76 | 98 ± 90 | 0.742 |
The preprocedural clinical and angiographic parameters were tested for their univariate and multivariate predictive value for the success of thrombus retrieval after TA. The variables included were preprocedural Thrombolysis In Myocardial Infarction flow grade, vessel reference diameter, minimum lumen diameter, right coronary artery, and left circumflex artery. On multivariate analysis, the significant predictors of successful thrombus retrieval were right coronary artery and vessel reference diameter, with an OR of 4.11 (95% CI 1.45 to 11.6, p = 0.007) and 1.52 (95% CI 0.94 to 2.85, p = 0.05), respectively.
The CE-MRI results are summarized in Table 3 . Initial and follow-up CE-MRI studies were available for all patients. The median interval between STEMI presentation and CE-MRI scans performed in the acute phase and at follow-up were similar in the 2 groups. No difference in acute phase left ventricular ejection fraction, volumes, or mass was observed between the 2 groups. We found a favorable, but not significant, trend toward reduced left ventricular remodeling in the TA-positive group.
| Variable | Acute Phase TA | 6-Mo Follow-up TA | ||||
|---|---|---|---|---|---|---|
| No (n = 50) | Yes (n = 38) | p Value | No (n= 50) | Yes (n = 38) | p Value | |
| Interval to scan (days) | 0.657 | 0.423 | ||||
| Median | 5.2 | 5.4 | 195 | 188 | ||
| Range | 3–7 | 3–8 | 171–215 | 166–204 | ||
| Left ventricular end-diastolic volume (ml) | 114 ± 27 | 108 ± 22 | 0.332 | 115 ± 27 | 108 ± 25 | 0.159 |
| Left ventricular end-systolic volume (ml) | 55 ± 22 | 58 ± 15 | 0.460 | 55 ± 25 | 54 ± 17 | 0.750 |
| Change in left ventricular end-diastolic volume (%) | — | — | — | −0.3 ± 16 | −3.7 ± 16 | 0.328 |
| Change in left ventricular end systolic volume (>20%) | — | — | — | 4 (8.0) | 2 (5.2) | 0.298 |
| Left ventricular mass (g) | 13 ± 2 | 144 ± 35 | 0.103 | 131 ± 19 | 141 ± 36 | 0.201 |
| Left ventricular ejection fraction | 52 ± 11% | 50 ± 10% | 0.362 | 52 ± 12% | 49 ± 10% | 0.301 |
| Infarct size transmurality | 29 (58%) | 18 (47%) | 0.423 | 28 (56%) | 18 (47%) | 0.556 |
| Infarct size (g) | 37 ± 21 | 22 ± 11 | 0.001 | 29 ± 19 | 17 ± 10 | 0.005 |
| Infarct size (%) | 28 ± 15 | 15 ± 9 | 0.004 | 22 ± 14 | 12 ± 8 | 0.002 |
| Microvascular obstruction | 31 (62%) | 19 (50%) | 0.284 | — | — | — |
| Early microvascular obstruction (g) | 10.3 ± 3.7 | 5.6 ± 3.1 | 0.007 | — | — | — |
| Early microvascular obstruction | 7.6 ± 2.1% | 3.8 ± 1.1% | 0.003 | — | — | — |
| Late microvascular obstruction (g) | 5.4 ± 2.9 | 2.1 ± 0.9 | 0.007 | — | — | — |
| Late microvascular obstruction | 5.4 ± 2.9% | 2.1 ± 0.6% | 0.006 | — | — | — |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


