The role of low-dose thrombolysis in the reduction of pulmonary artery pressure in moderate pulmonary embolism (PE) has not been investigated. Because the lungs are very sensitive to thrombolysis, we postulated that effective and safe thrombolysis might be achieved by a lower dose of tissue plasminogen activator. The purpose of the present study was to evaluate the role of this “safe dose” thrombolysis in the reduction of pulmonary artery pressure in moderate PE. During a 22-month period, 121 patients with moderate PE were randomized to receive a “safe dose” of tissue plasminogen activator plus anticoagulation (thrombolysis group [TG], n = 61 patients) or anticoagulation alone (control group [CG], n = 60). The primary end points consisted of pulmonary hypertension and the composite end point of pulmonary hypertension and recurrent PE at 28 months. Pulmonary hypertension and the composite end point developed in 9 of 58 patients (16%) in the TG and 32 of 56 patients (57%) in the CG (p <0.001) and 9 of 58 patients (16%) in the TG and 35 of 56 patients (63%) in the CG (p <0.001), respectively. The secondary end points were total mortality, the duration of hospital stay, bleeding at the index hospitalization, recurrent PE, and the combination of mortality and recurrent PE. The duration of hospitalization was 2.2 ± 0.5 days in the TG and 4.9 ± 0.8 days in the CG (p <0.001). The combination of death plus recurrent PE was 1 (1.6%) in TG and 6 (10%) in the CG (p = 0.0489). No bleeding occurred in any group, and despite a positive trend in favor of a “safe dose” thrombolysis, no significant difference was noted in the rate of individual outcomes of death and recurrent PE when assessed independently. In conclusion, the results from the present prospective randomized trial suggests that “safe dose” thrombolysis is safe and effective in the treatment of moderate PE, with a significant immediate reduction in the pulmonary artery pressure that was maintained at 28 months.
Thrombolysis is an effective tool in the treatment of massive pulmonary embolism (PE). It has also been recommended for “submassive PE,” in which hemodynamic stability is present but with right ventricular (RV) enlargement or hypokinesia or the elevation of biomarkers of RV injury. The dreaded complication of thrombolysis is intracerebral hemorrhage, which has been noted in 0.7% to 6.4% of patients receiving thrombolysis. This frequency, albeit low, has caused a reluctance in the use of thrombolysis for symptomatic PE without hemodynamic instability. Our experience with percutaneous endovenous intervention for deep venous thrombosis has suggested an exquisitely favorable pulmonary response to low-dose thrombolysis. The lungs are uniquely sensitive to thrombolysis, because they are the only organ receiving the entire cardiac output. Furthermore, they are the point of convergence for the entire molecules of the thrombolytic agent, no matter through which vein the drug is administered. It is therefore intriguing to postulate that a lower dose of the thrombolytic drug might be effective in PE, with the additional benefit of enhancing its safety profile. No data are available on peripheral intravenous administration of low-dose thrombolysis for “moderate” PE in the reduction of pulmonary artery pressures after 2 years. The present study was, therefore, undertaken to assess the effects of low-dose tissue plasminogen activator (tPA) on pulmonary artery systolic pressure in patients with “moderate” PE at 28 months.
Methods
The Moderate Pulmonary Embolism Treated with Thrombolysis trial was a prospective, controlled, randomized, single-center open study that enrolled 121 adult patients with symptomatic “moderate” PE. All patients provided written informed consent, and the institutional review board approved the study protocol.
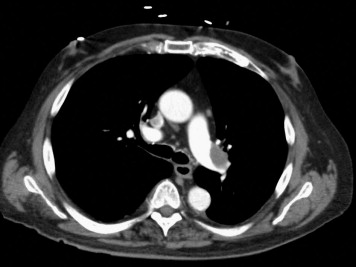
Adult patients presenting with signs and symptoms suggestive of PE plus imaging documentation on computed tomographic angiography or ventilation/perfusion scanning were potentially eligible for the study. “Moderate” PE was defined as the presence of signs and symptoms of PE plus computed tomographic pulmonary angiographic involvement of >70% involvement of thrombus in ≥2 lobar or left or right main pulmonary arteries ( Figure 1 ) or by a high probability ventilation/perfusion scan showing ventilation/perfusion mismatch in ≥2 lobes. Interpretation of the radiologic findings were performed by radiologists not participating in the study. To be eligible for enrollment, the patients were required to have a minimum of ≥2 new signs and symptoms consisting of chest pain, tachypnea (respiratory rate at rest ≥22 breaths/min), tachycardia (heart rate at rest ≥90 beats/min), dyspnea, cough, oxygen desaturation (oxygen partial pressure <95% ) or elevated jugular venous pressure ≥12 cm H 2 O. RV enlargement or hypokinesia and elevation of biomarkers of RV injury (troponin I and brain natriuretic peptide), although measured, were not a requirement for enrollment. The exclusion criteria included an onset of symptoms >10 days; >8 hours since the start of parenteral anticoagulation; systemic arterial systolic blood pressure <95 or ≥200/100 mm Hg; eligibility for full-dose thrombolysis; a contraindication to unfractionated or low-molecular-weight heparin; severe thrombocytopenia (platelet count <50,000/mm 3 ); major bleeding within <2 months requiring transfusion; surgery or major trauma within <2 weeks; brain mass; neurologic surgery, intracerebral hemorrhage, or subdural hematoma within <1 year; end-stage illness with no plan for PE treatment; and an inability to perform echocardiography because of chest deformities, bandages, or catheters.
The primary end points of the study were the development of pulmonary hypertension as assessed by echocardiography and the composite end point of pulmonary hypertension and recurrent PE at intermediate-term follow-up. The results were adjudicated after a mean follow-up of 28 ± 5 months. The secondary end points were total mortality, duration of hospital stay and bleeding at index hospitalization, recurrent PE, and the composite end points of mortality and recurrent PE.
Echocardiography was performed within 2 hours after randomization and before administration of tPA and was repeated 24 to 48 hours after and at 6-month intervals. Pulmonary artery systolic pressure was estimated from the tricuspid valve regurgitant jet velocity using the modified Bernoulli equation 4v 2 + right atrial pressure. The right atrial pressure was estimated at 10, 15, and 18 mm Hg for mild, moderate, and severe right atrial enlargement, respectively. In the standard 4-chamber view if the maximum dimension of the right atrium/left atrium was 1 to 1.2, right atrial enlargement was arbitrarily considered as mild, 1.3 to 1.5 as moderate, and >1.5 as severe. In the latter case, the diameter of the inferior vena cava had to be ≥2.5 cm as an additional requirement to assign a value of 18 mm Hg for the right atrial pressure, otherwise it was still considered moderate, right atrial enlargement. If the right atrial size was less than that of the left atrium, the right atrial pressure was assumed to be 5 mm Hg. Interpretation of the echocardiographic findings was performed by a cardiologist who was unaware of the patients’ treatment assignments and using digitized videos. A dichotomous value of pulmonary artery systolic pressure of ≥40 mm Hg was used to define pulmonary hypertension. Right ventricular enlargement was defined as an RV/left ventricular ratio of >0.9. The videos were also evaluated for RV hypokinesia, defined as a reduction in the anticipated normal wall motion of the RV myocardium.
All patients received either unfractionated heparin or subcutaneous enoxaparin, with initial preference given to the latter drug. Enoxaparin was given to 48 of 61 (79%) of the patients in the thrombolysis group (TG) and 49 of 60 (81%) in the control group (CG). Administration of unfractionated heparin was determined by the presence of renal insufficiency or patient preference. In the TG, enoxaparin was given as 1 mg/kg subcutaneously twice daily, with the initial dose not to exceed 80 mg. For unfractionated heparin in the same group, it was given at 70 U/kg as a bolus but not to exceed 6,000 U, with subsequent dose adjustment to keep the activated partial thromboplastin time at 1.5 to 2 times the baseline value. Although tPA was infused, the maintenance dose of unfractionated heparin was kept at 10 U/kg/hour and not to exceed 1,000 U/hour. At 3 hours after termination of thrombolysis, the dose of unfractionated heparin was increased to 18 U/kg/hour. In the CG, enoxaparin was given at 1 mg/kg subcutaneously twice daily and unfractionated heparin at 80 U/kg as a bolus followed by 18 U/kg/hour, with the same partial thromboplastin time target.
In the present study, tPA was the only thrombolytic drug used. The dose of tPA was ≤50% of the standard dose (100 mg) commonly used for the treatment of PE, which we termed “safe dose” thrombolysis. For patients weighing ≥50 kg, the total dose was 50 mg, which was given as a 10-mg bolus by an intravenous push within 1 minute followed by infusion of the remaining 40 mg within 2 hours. For those weighing <50 kg, the total dose was calculated as 0.5 mg/kg, which was given as a 10-mg initial bolus followed by the remainder within 2 hours. Warfarin was started at admission in all patients.
A significant paucity of data is available on the changes of pulmonary artery systolic pressure from before treatment to intermediate follow-up in patients with moderate PE, who have received thrombolysis versus anticoagulation alone. In 1 study, there was a 55% reduction in the RV systolic pressure at 6 months in patients receiving 100 mg of tPA versus only a 15% reduction in those treated with anticoagulation alone, leading to a difference in reduction of 40%. In another study of 12 patients, who had received urokinase or streptokinase, the mean pulmonary artery pressure decreased from 28 to 17 mm Hg in 7.5 years (39% reduction). In the 11 patients, who received heparin only, the decrease in pressure was from 26 to 22 mm Hg in 7.3 years (15% reduction), leading to a difference in the reduction of 24%. We assumed a lower change and to be conservative, anticipated a difference in the percentage of reduction of 10. To show the significance of this difference with a power of 90% and a 2-sided α of 0.05, and an estimated SD of 9, 55 patients were required in each group. We increased this number to 60 for an estimated 10% attrition rate. A comparison between the 2 groups was made using unpaired t test for continuous variables and Fisher’s exact test for categorical variables (2-tailed). The data are expressed as mean ± SD. Statistical analyses were performed with Statistica software, version 10 (StatSoft, Tulsa, Oklahoma).
Results
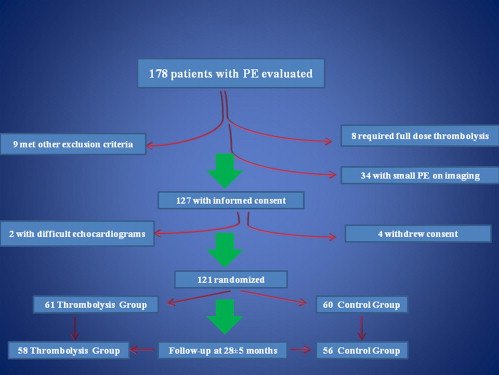
During a 22-month period from May 2008, 178 patients with PE were considered for enrollment in the present study. The patient flow is shown in Figure 2 . Ultimately, 121 patients were randomized. The randomization process occurred after the inclusion and exclusion criteria were satisfied, and the patients had provided written informed consent. After evaluation of the patient, the study investigator placed a telephone call to the study center, and, by opening of sealed envelopes, randomization to the TG or CG was made. A total of 61 patients were randomized to the TG and 60 to the CG. The baseline clinical characteristics were similar between the 2 groups ( Table 1 ). Follow-up was obtained for 58 patients in the TG and 56 in the CG. The mean follow-up period was 28 ± 5 months. The primary and secondary end points are listed in Tables 2 and 3 , respectively.
| Variable | TG (n = 61; 100%) | CG (n = 60; 100%) | p Value |
|---|---|---|---|
| Men | 28 (46%) | 27 (45%) | 0.92 |
| Age (yrs) | 58 ± 9 | 59 ± 10 | 0.56 |
| Weight (kg) | 84 ± 14 | 83 ± 13 | 0.68 |
| Previous or concomitant disease | |||
| Hypertension | 32 (52%) | 31 (52%) | 0.93 |
| Diabetes mellitus | 23 (38%) | 25 (40%) | 0.66 |
| Cardiovascular | 35 (57%) | 37 (62%) | 0.80 |
| Hypercholesterolemia ∗ | 27 (33%) | 25 (30%) | 0.77 |
| Pulmonary | 22 (36%) | 25 (42%) | 0.53 |
| Renal | 8 (13%) | 9 (15%) | 0.77 |
| Current smoker | 12 (20%) | 15 (25%) | 0.48 |
| Unprovoked pulmonary embolism | 28 (46%) | 27 (45%) | 0.92 |
| Estrogen therapy | 6 (10%) | 7 (12%) | 0.75 |
| Cancer | |||
| Active | 8 (13%) | 9 (15%) | 0.77 |
| History | 3 (5%) | 3 (5%) | 0.98 |
| Known prothrombotic state | 6 (10%) | 5 (8%) | 0.77 |
| Previous venous thromboembolism | 13 (21%) | 12 (20%) | 0.86 |
| Concomitant deep venous thrombosis | 35 (57%) | 33 (55%) | 0.79 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


