We studied the effect of the preprocedural intravascular ultrasound findings on stent expansion and the pre- and postprocedural findings on the long-term clinical outcomes in patients undergoing drug-eluting stent implantation for unprotected left main (LM) bifurcation disease. Using a left anterior descending (LAD) pullback, we evaluated the ostial LAD artery (3 mm distal to the carina), the polygon of confluence (POC; the confluent zone of the LAD artery and left circumflex artery), and the distal LM artery (3 mm just proximal to the POC). The measurements included the minimum lumen area (MLA) and minimum stent area within each segment. In 168 LM bifurcations, the preprocedural MLA and post-stenting minimum stent area within the LM artery were located within the POC in 41% and 70%, respectively. Independent predictors for the post-stent minimum stent area within the distal portion of LM artery above the LAD carina were the preprocedural lumen area of the LAD carina (β = 0.253, 95% confidence interval [CI] 0.10 to 0.36, p = 0.001) and preprocedural MLA within the POC (β = 0.205, 95% CI 0.04 to 0.23, p = 0.008). During the 41.8 ± 18.0-month follow-up period, 26 patients experienced cardiac events. In the multivariate Cox model, female gender (adjusted hazard ratio 2.56, 95% CI 1.173 to 5.594, p = 0.018) and preprocedural MLA within the POC (adjusted hazard ratio 0.829, 95% CI 0.708 to 0.971, p = 0.020) were independent predictors for the occurrence of events at 3 years of follow-up. In conclusion, as assessed by the LAD pullback, the preprocedural MLA within the POC was a surrogate reflecting the overall severity of LM bifurcation disease, contributed to the post-stent minimum stent area within the distal segment of LM bifurcation, and was a predictor of clinical events during follow-up.
Drug-eluting stent implantation for unprotected left main (LM) coronary artery stenosis has been evolving into a feasible therapeutic alternative to bypass surgery, especially in patients at very high surgical risk. However, irrespective of the stent implantation strategy, the LM bifurcation location is a major determinant of adverse outcomes, even in the drug-eluting stent era. The intravascular ultrasound (IVUS) examination can provide unique insights into the extent and distribution of coronary atherosclerosis. However, no data are available suggesting the pre- and postprocedural IVUS predictors of adverse events after drug-eluting stent implantation into distal LM bifurcation stenoses. The aims of the present study were to assess the distribution patterns of atherosclerotic plaque and the severity of bifurcation LM stenosis and the effect of the IVUS lesion characteristics on stent expansion and the long-term clinical outcomes in patients undergoing drug-eluting stent implantation for unprotected distal bifurcation LM disease.
Methods
From February 2003 to November 2007, 509 patients with unprotected LM disease (angiographic diameter stenosis >50%) underwent percutaneous coronary intervention with drug-eluting stent implantation at the Asan Medical Center (Seoul, Korea). Of these 509 patients, 168 with distal LM bifurcation lesions underwent preprocedural IVUS obtained by pullback from the left anterior descending artery (LAD) to the LM (LAD pullback). All patients had immediate post-stenting LAD pullback images available.
All patients underwent either routine angiographic surveillance at 6 to 12 months or clinically driven repeat angiography (follow-up period 16.6 ± 15.5 months). After the angiographic follow-up examination, noninvasive stress testing was performed annually. Inducible ischemia on a stress test (with or without ischemic chest pain) was regarded as a clinical indicator for angiography. The clinical follow-up period was 42.0 ± 17.8 months. Major adverse cardiovascular events were defined as death from cardiac causes, target lesion revascularization, and myocardial infarction. Myocardial infarction was diagnosed by the presence of ischemic symptoms or signs plus cardiac enzyme elevation (creatine kinase-MB elevation >3 times or creatine kinase elevation >2 times the upper limit of normal). All patients provided written informed consent, and the ethics committee approved the present study.
Qualitative and quantitative angiographic analysis was done using standard techniques with automated edge-detection algorithms (CASS-5, Pie-Medical B.V., Maastricht, The Netherlands) in the angiographic analysis center of the Cardiovascular Research Foundation (Seoul, Korea). Angiographic stenosis was defined as >50% diameter stenosis. At follow-up, in-stent restenosis was defined as >50% diameter stenosis anywhere within the LM artery or within 5 mm distal to the LAD artery or left circumflex artery ostia. The Medina classification was used to describe the location and distribution of lesions at the LM bifurcation.
IVUS imaging was performed after intracoronary administration of 0.2 mg nitroglycerin using motorized transducer pullback (0.5 mm/s) and a commercial scanner (Boston Scientific/SCIMED, Minneapolis, Minnesota) consisting of a rotating 30- or 40-MHz transducer within a 3.2Fr imaging sheath. Using computerized planimetry (EchoPlaque, version 3.0, Indec Systems, MountainView, California), off-line IVUS analysis was performed.
The carina was identified as the frame immediately distal to the take-off of the side branch. In the present study, most patients did not undergo left circumflex artery pullback. Therefore, on the LAD pullback, 3 segments within the LM bifurcation were defined ( Figure 1 ): the ostial LAD (3 mm distal to the carina), the polygon of confluence (POC; confluence zone of the LAD and left circumflex artery on longitudinal IVUS image reconstruction in parallel with the quantitative coronary analysis-based definition suggested by Ramcharitar ), and the distal LM (3 mm just proximal to the POC). At the site of the minimum lumen area (MLA) within each of these 3 segments, the cross-section areas of lumen, stent, and external elastic membrane were measured using 2-dimensional planimetry. The plaque burden was calculated as the plaque + media/external elastic membrane × 100%. The IVUS criteria for a significant stenosis was an MLA <4.0 mm 2 at the LAD ostium and an MLA <6.0 mm 2 at the distal LM (including the distal LM and POC).
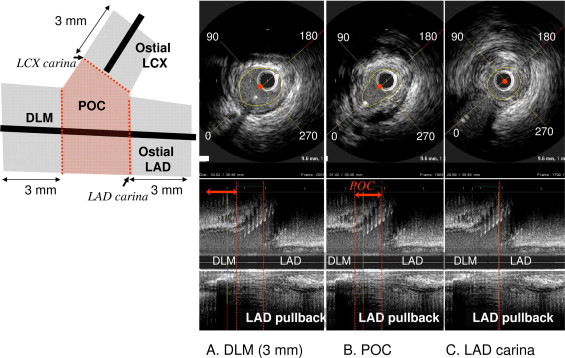
The circumferential plaque distribution was assessed at the MLA sites of each segment. Using a protractor centered on the lumen, the arc of the circumferential distribution of significant plaque (>0.6-mm plaque thickness) was measured in a clockwise direction using the take-off of the side branch as 0° ( Figure 1 ).
Immediately after stenting, 3 segments (distal LM, POC, and LAD ostium) were identified in parallel with the preprocedural assessments. The minimum stent area was measured within each segment. In addition, the minimum stent area within the distal portion of LM above the LAD carina (to include both the distal LM and the POC) was tabulated. In lesions treated with a kissing technique, the minimum stent area within the POC included both stents ( Figure 2 ).
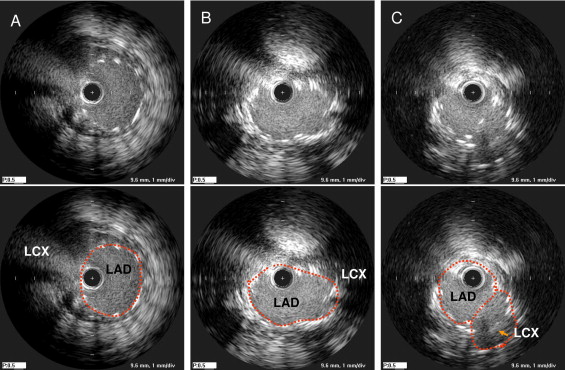
A post-stenting left circumflex artery pullback IVUS examination was performed in 66 lesions. The post-stenting MLA in the single-stent group and the post-stenting minimum stent area in the 2-stent group were measured <3 mm distal to the carina.
All statistical analyses were performed using Statistical Analysis Systems, release 9.1 (SAS Institute, Cary, North Carolina), or the Statistical Package for Social Sciences, version 10.0 (SPSS, Chicago, Illinois). All values are presented as the mean ± SD for continuous variables or counts and percentages for categorical variables. Continuous variables were compared using the unpaired t test or nonparametric Mann-Whitney U test. Categorical variables were compared using the chi-square statistics or Fisher’s exact test.
Stepwise multiple linear regression analysis was performed to assess the independent predictors for the post-stenting minimum stent area of the distal LM and LAD ostium. Cox proportional hazard regression analyses were performed to find the predictors of long-term adverse outcomes. Variables with p ≤0.20 on the univariate analyses were candidates for the multivariate Cox proportional hazard regression models. A backward elimination process was used to develop the final multivariate model, and the adjusted hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated. The proportional hazards assumption was confirmed by testing of partial (Schoenfeld) residuals, and no relevant violations were found. A p value <0.05 was considered statistically significant.
Results
The baseline clinical and procedural characteristics in 168 LM bifurcations are listed in Table 1 . The most common types, using the Medina classification, were (1,1,1) in 71 (45%), (1,1,0) in 48 (30%), (0,1,0) in 16 (10%), and (1,0,0) in 10 (6%).
| Variable | Bifurcating LM |
|---|---|
| Age (years) | 59.4 ± 10.8 |
| Men | 126 (75%) |
| Hypertension | 83 (49%) |
| Diabetes mellitus | 54 (32%) |
| Smoker | 54 (32%) |
| Hyperlipidemia ⁎ | 66 (39%) |
| Clinical manifestation | |
| Stable angina pectoris | 85 (51%) |
| Unstable angina pectoris | 81 (48%) |
| Acute myocardial infarction | 2 (1%) |
| Glycoprotein IIb/IIIa | 21 (13%) |
| Intra-aortic balloon pump | 11 (7%) |
| Drug-eluting stent type | |
| Cypher | 161 (96%) |
| Taxus | 6 (4%) |
| Stent length (mm) | 40.7 ± 22.0 |
| Stent diameter (mm) | 3.4 ± 0.2 |
| Maximum balloon pressure (atm) | 17.5 ± 3.1 |
| Types of 2-stenting techniques | |
| T stenting | 10 (15%) |
| Crush stenting | 37 (54%) |
| Kissing stents | 21 (30%) |
| Culotte technique | 1 (1%) |
⁎ Defined as total cholesterol >200 mg/dl or taking antilipidemic medication.
A comparison of the angiographic data and IVUS-defined stenoses is listed in Table 2 . In both the distal LM and the LAD ostium, the sensitivity of an angiographically defined diameter stenosis >50% to predict for IVUS stenosis was high (97% in the distal LM and 96% in the ostial LAD). In contrast, the specificity was low (33% in the distal LM and 41% in the ostial LAD).
| Angiographic Diameter Stenosis | IVUS-Defined Minimum Lumen Area (mm 2 ) | |
|---|---|---|
| Distal left main ⁎ | ≥6.0 | <6.0 |
| ≤50% | 23 | 4 |
| >50% | 33 | 108 |
| Ostial left anterior descending † | ≥4.0 | <4.0 |
| ≤50% | 25 | 4 |
| >50% | 51 | 89 |
⁎ Sensitivity 97%, specificity 33%, positive predictive value 64%, and negative predictive value 89%.
† Sensitivity 96%, specificity 41%, positive predictive value 77%, and negative predictive value 85%.
The preintervention IVUS findings of the 168 LM bifurcations are listed in Table 3 . The length of the POC was 3.3 ± 1.3 mm on the longitudinal IVUS image reconstruction and analysis. The MLA of the LM above the LAD carina was located within the POC in 68 lesions (41%) and within the distal LM in 100 (59%). The MLA within the POC correlated positively with the MLA within the distal LM (r = 0.73, p <0.001), the plaque burden of the distal LM (r = −0.63, p <0.001), the lumen area within the LAD carina (r = 0.31, p <0.001), and the plaque burden of the LAD carina (r = −0.28, p <0.001).
| IVUS Findings | Value |
|---|---|
| Preprocedural | |
| At minimum lumen area site of ostial left anterior descending artery | |
| Lumen area (mm 2 ) | 4.0 ± 2.1 |
| External elastic membrane area (mm 2 ) | 12.6 ± 3.5 |
| Plaque burden (%) | 66.9 ± 13.9 |
| At carina of LAD | |
| Lumen area (mm 2 ) | 4.5 ± 2.3 |
| External elastic membrane area (mm 2 ) | 13.4 ± 3.8 |
| Plaque burden (%) | 65.7 ± 14.5 |
| Minimum lumen of polygon of confluence (mm 2 ) | 6.4 ± 3.1 |
| At minimum lumen area of distal left main | |
| Lumen area (mm 2 ) | 5.8 ± 3.0 |
| External elastic membrane area (mm 2 ) | 18.7 ± 5.5 |
| Plaque burden (%) | 68.2 ± 13.9 |
| Post-stenting | |
| Minimal stent area within polygon of confluence (mm 2 ) | 8.5 ± 2.3 |
| Minimal stent area within distal left main (mm 2 ) | 9.8 ± 2.7 |
| Minimal stent area within distal portion of left main above carina (mm 2 ) | 8.1 ± 2.0 |
| Minimal stent area within ostial left anterior descending artery (mm 2 ) | 7.5 ± 1.8 |
| Minimal stent area within ostial left circumflex artery (mm 2 ) ⁎ (n = 66) | 6.1 ± 2.1 |
⁎ Data obtained from left circumflex artery pullback in 66 lesions; minimum lumen area was equivalent to minimum stent area in 2-Stent group.
The 79 lesions with significant preprocedural angiographic stenosis of the left circumflex artery ostium had a smaller MLA within the POC than did the lesions without significant angiographic ostial left circumflex artery narrowing (5.4 ± 2.5 vs 7.5 ± 3.4 mm 2 , p <0.001). Similarly, the MLA within the distal LM was smaller (5.4 ± 2.8 vs 6.6 ± 3.2 mm 2 , p = 0.011) and the plaque burden greater (70.5 ± 13.1% vs 64.7 ± 13.7%, p = 0.007) for lesions with compared to those without angiographic ostial left circumflex artery narrowing.
At the LAD ostium, significant plaque (>0.6 mm thickness) was mostly located opposite the flow divider (90° to 270°) in 155 lesions (92%). However, circumferential plaque (>0.6 mm thickness) was observed at the MLA site within the POC in 86 lesions (51%). Angiographic stenosis of the left circumflex artery ostium was seen in 51 (59%) of 86 lesions with circumferential POC plaque compared to 33 (40%) of 82 lesions without circumferential POC plaque (p = 0.014). Lesions with circumferential POC plaque showed a significantly smaller prepercutaneous coronary intervention MLA within the POC (5.4 ± 2.5 vs 7.4 ± 3.4 mm 2 , p <0.001) and the MLA within the distal LM (5.2 ± 2.5 vs 6.6 ± 3.3 mm 2 , p = 0.002) compared to the lesions with noncircumferential POC plaque.
The post-stenting minimum stent area within the distal portion of LM above the LAD carina was located within the POC in 117 (70%) of 168 lesions. The postprocedural IVUS findings are summarized in Table 3 , and Table 4 lists the pre- and post-stenting IVUS findings according to the different stenting techniques.
| Techniques | Patients (n) | Preprocedural MLA Within | Post-Stenting MLA Within | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Distal LM (mm 2 ) | POC (mm 2 ) | LAD Ostium (mm 2 ) | Left Circumflex Artery Ostium (mm 2 ) ⁎ | Distal LM (mm 2 ) | POC (mm 2 ) | LAD Ostium (mm 2 ) | Left Circumflex Artery Ostium (mm 2 ) † | ||
| Single stent | 99 | 6.2 ± 3.2 | 7.1 ± 3.3 | 4.4 ± 2.1 | 6.1 ± 3.1 | 9.6 ± 2.4 | 8.5 ± 2.2 | 7.8 ± 1.9 | 7.5 ± 2.6 |
| Two stent | 69 | 5.3 ± 2.7 | 5.3 ± 2.6 ‡ | 3.6 ± 2.0 ‡ | 3.6 ± 1.4 ‡ | 10.0 ± 3.0 | 8.5 ± 2.6 | 7.5 ± 2.0 | 5.7 ± 1.8 ‡ |
| T-stent | 10 | 5.6 ± 2.4 | 4.6 ± 2.2 ‡ | 3.3 ± 1.1 | 3.0 ± 1.4 ‡ | 10.2 ± 1.9 | 6.7 ± 1.6 ‡ § | 7.8 ± 1.4 § | 5.0 ± 2.5 ‡ |
| Crush | 37 | 5.3 ± 2.8 | 5.4 ± 2.9 ‡ | 3.8 ± 2.5 ‡ | 3.7 ± 1.8 ‡ | 10.1 ± 3.4 | 7.6 ± 1.6 ‡ § | 7.3 ± 1.8 | 6.1 ± 1.8 |
| Kissing | 21 | 5.1 ± 3.1 | 5.3 ± 2.1 ‡ | 3.5 ± 1.2 | 3.7 ± 1.5 ‡ | 9.8 ± 3.0 | 11.1 ± 2.5 ‡ | 6.4 ± 1.4 ‡ | 5.5 ± 1.2 ‡ |
| Culotte | 1 | 5.7 | 7.0 | 2.6 | 3.20 | 13.0 | 6.6 | 7.5 | 5.2 |
| Total | 168 | 5.8 ± 3.0 | 6.4 ± 3.1 | 4.0 ± 2.1 | 4.5 ± 2.5 | 9.8 ± 2.7 | 8.5 ± 2.3 | 7.5 ± 1.8 | 6.1 ± 2.1 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


