Angiotensin II and aldosterone are key factors responsible for the structural and neurohormonal remodeling of the atria and ventricles in patients with atrial fibrillation (AF). The aim of the present study was to evaluate the antiarrhythmic effects of spironolactone compared to angiotensin-converting enzyme inhibitors in patients with recurrent AF. A cohort of 164 consecutive patients (mean age 66 years, 87 men), with an average 4-year history of recurrent AF episodes, was enrolled in a prospective, randomized, 12-month trial with 4 treatment arms: group A, spironolactone, enalapril, and a β blocker; group B, spironolactone and a β blocker; group C, enalapril plus a β blocker; and group D, a β blocker alone. The primary end point of the trial was the presence of symptomatic AF episodes documented on the electrocardiogram. At 3-, 6-, 9-, and 12 months, a significant (p <0.001) reduction had occurred in the incidence of AF episodes in both spironolactone-treated groups (group A, spironolactone, enalapril, and a β blocker; and group B, spironolactone plus a β blocker) compared to the incidence in patients treated with enalapril and a β blocker (group C) or a β blocker alone (group D). No significant difference was seen in AF recurrences between patients taking spironolactone and a β blocker with (group A) and without (group B) enalapril. No significant differences were found in the systolic or diastolic blood pressure or heart rate among the groups before and after 1 year of follow-up. In conclusion, combined spironolactone plus β-blocker treatment might be a simple and valuable option in preventing AF episodes in patients with normal left ventricular function and a history of refractory paroxysmal AF.
To date, no prospective trials of aldosterone antagonists in patients with atrial fibrillation (AF) have been published. The aim of the present prospective, randomized, clinical trial was to evaluate the antiarrhythmic effects of spironolactone combined with enalapril and a β blocker or with a β blocker compared to treatment with enalapril and a β blocker or a β blocker alone in patients with a history of symptomatic paroxysmal and persistent AF.
Methods
The study was supported by the State Committee for Scientific Research and the Institute of Cardiology grant and registered (Institute of Cardiology, 2.8/IV/2005). Bioethical Committee approval was issued on December 7, 2004 (registration number 865). No conflict of interest or any financial or personal relation with other persons or organizations was present that could have inappropriately influenced our work.
The study was designed as a prospective, randomized, open-label clinical trial and was conducted from January 2005 to January 2009 in one center. The studied population consisted of consecutive patients seen in the hospital and outpatient clinic because of paroxysmal AF episodes without structural heart disease. The inclusion criteria were as follows: age 40 to 80 years, sinus rhythm at enrollment, a history of symptomatic paroxysmal AF, with evidence of ≥2 electrocardiographic-confirmed episodes of AF, patients with an awareness of AF episodes because of a history of AF, and a known intolerance and low efficacy of antiarrhythmic drugs (recurrence of AF events) from class I (propafenone), III (sotalol, amiodarone), and IV (verapamil, diltiazem) drugs according to the Vaughan-Williams classification. No other antiarrhythmic drugs are available on the local market. The patients had not been treated with angiotensin-converting enzyme inhibitors (ACE-I), angiotensin receptor blockers, and aldosterone antagonists before enrollment. The previous treatment of concomitant diseases was not changed. Antithrombotic treatment was conducted according to the current standards and the Congestive heart failure, Hypertension, Age, Diabetes, Stroke (CHADS 2 ) scale. Every patient was treated with β blockers to control the heart rate. The exclusion criteria were as follows: asymptomatic forms of AF, unstable arterial hypertension, angina greater than Canadian Cardiovascular Society (CCS II), myocardial infarction during the previous 6 months, heart failure (New York Heart Association class I to IV), contraindications to ACE-I and spironolactone, renal insufficiency (serum creatinine >1.2 mg/dl), structural heart disease, prosthetic valves, left ventricle ejection fraction <55%, and inflammatory disease.
During the first visit, after the informed consent process, a detailed questionnaire of the medical history and an electrocardiographic and 2-dimensional echocardiographic examination were performed. Conventional M-mode, 2-dimensional, and Doppler echocardiography examinations were performed using the Sonos 5000 (Philips Medical Systems, Andover, Massachusetts). Three consecutive measurements were obtained from the classic views by an experienced cardiologist who was unaware of the treatment assignment. The ejection fraction was estimated using the Simpson biplane method. Blood samples were taken to determine the blood count, including the white blood cell count and smear (to exclude an inflammatory process), creatinine, and electrolytes. To exclude a potential influence of the treatment on renal function, the blood concentration of potassium, sodium, and creatinine was examined again 2 weeks after randomization and after 12 months. The echocardiographic examinations were repeated after 12 months.
The patients were randomized to 4 treatment arms: group A, a β blocker, spironolactone, and enalapril; group B, a β blocker and spironolactone; group C, a β blocker and enalapril; and group D, a β blocker alone (control group). The dose of β blocker (propranolol, metoprolol, or bisoprolol) was titrated to achieve a heart rate at rest of 60 to 70 beats/min in sinus rhythm. The spironolactone dose was 25 mg; the mean dose of enalapril was 12.5 mg (minimum 10 mg/day). In hypertensive patients, blood pressure was controlled to achieve a level <130/80 mm Hg. Diuretics, dihydropiridine calcium antagonists, and/or α blockers were added, if necessary, to control the blood pressure. No changes were made to the statin therapy before or during the study, with 59% of patients continuing treatment with statins at the same doses in each treatment group (p = NS). No long-term oral antiarrhythmic drugs were used. Short-time interventions during the AF episodes were performed as needed to restore sinus rhythm using intravenous amiodarone, propafenone, or electrical cardioversion.
The number of symptomatic episodes of AF, confirmed by electrocardiographic, emergency department, and hospital stay records, during the previous 3 months before the baseline visit were collected from every patient and served as a reference period for the trial. The studied patients had usually been receiving routine care at our AF clinic for a few years and were familiar with the need to document any episodes of AF. The study visits were conducted every 3 months, with detailed reports of AF occurrence, all electrocardiograms were collected, and electrocardiography was performed. The patients were instructed to keep diaries and to visit the hospital emergency department if symptoms occurred suggestive of, or associated with, arrhythmia. AF episodes that were >1 hour and had been confirmed by electrocardiography were recorded ( Figures 1 and 2 ) .
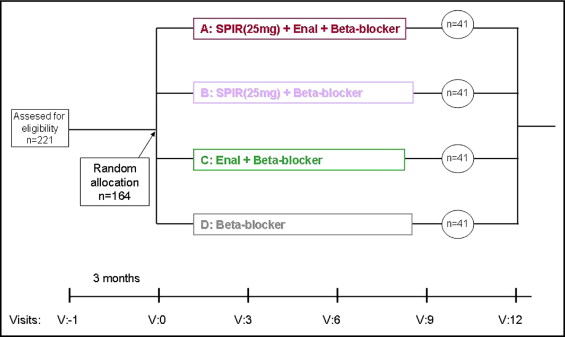
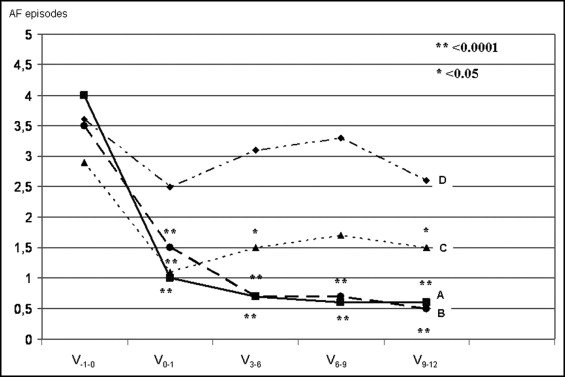
The primary outcome was the incidence of symptomatic AF episodes for 12 months, documented every 3 months, compared to the baseline number of symptomatic, electrocardiographically confirmed AF episodes from the patients’ history during the 3 months before randomization. The secondary outcome was evaluation of the tolerance and safety of spironolactone treatment.
Continuous variables are reported as mean values and standard deviations, if normally distributed, or as median values and the interquartile range. The Shapiro-Wilk test was performed to assess the normality of continuous variables. The t test and 2-way analysis of variance were used to compare differences in clinical parameters among the different groups. Categorical variables are reported as the absolute numbers and percentages. We performed a chi-square test with Yates correction to evaluate the distribution of categorical data. The data were modeled as repeated analysis of variance to measure the differences between the AF episodes. Within-subject effects (time), between-subject effects (intervention—the fixed effects in the model), and interactions between the 2 types of effects (time × intervention) were estimated. The model included the (1) time variable “visit”; (2) intervention with spironolactone; (3) intervention with enalapril; (4) an interaction between the time and spironolactone effect; (5) an interaction between the time and enalapril effect; and (6) an interaction between time and the spironolactone and enalapril effect. Multivariate tests were performed (Wilks’ lambda, Pillai’s trace). Multiplication was applied to the number of AF episodes to minimize the correlation between the mean values and variance of the data. Because of the low dispersion from normality, to confirm our findings, we also applied Wilcoxon nonparametric tests. The statistical methods were verified with the assumption of a significance level at p <0.05. All analyzed tests were 2 sided. The sample size was calculated for analysis of variance with fixed effects to provide 90% study power for a 2-tailed α level of 0.05, assuming a root-mean-square standardized effect for the effects of 0.20 and for the interactions of 0.25. The calculated sample size was 160 subjects. In the present study, we achieved a power of 0.90 for ACE-I effect, 1.0 for spironolactone effect, and 0.96 for the interaction between enalapril and spironolactone. All statistical analyses were performed using the Statistical Analysis Systems software, version 9.02 (SAS Institute, Cary, North Carolina).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


