Most patients with chronic ischemia and an implantable cardiac defibrillator (ICD) for primary prevention do not experience therapies for ventricular arrhythmias on follow-up. The present study aimed to identify independent clinical, electrocardiographic, and echocardiographic predictors of death and occurrence of ICD therapy in patients with chronic ischemic cardiomyopathy and ICD for primary prevention. A total of 424 patients with chronic ischemic cardiomyopathy, ejection fraction ≤35%, and New York Heart Association (NYHA) class ≥II were recruited. All patients underwent echocardiography before ICD insertion. Primary outcome was all-cause mortality; secondary outcome was occurrence of appropriate ICD therapy on follow-up. Primary and secondary outcomes occurred in 84 and 95 patients, respectively. Patients who died were more likely to have diabetes (hazard ratio [HR] 1.67, 95% confidence interval [CI] 1.00 to 2.79, p = 0.049), higher NYHA class (HR 1.96, 95% CI 1.15 to 3.33, p = 0.013), lower peri-infarct strain on echocardiogram (HR 1.25, 95% CI 1.07 to 1.46, p = 0.005), and lower glomerular filtration rate (HR 1.01, 95% CI 1.00 to 1.03, p = 0.022). Only peri-infarct strain (HR 1.22, 95% CI 1.09 to 1.36, p <0.001) predicted the occurrence of ICD therapy on follow-up. In conclusion, in chronic ischemic patients with an ICD for primary prevention, the presence of diabetes, renal dysfunction, higher NYHA class, and impaired peri-infarct zone function were predictors of all-cause mortality. In contrast, only impaired peri-infarct zone function determined the occurrence of appropriate ICD therapy on follow-up.
Despite a decrease in the rate of death from cardiovascular diseases over recent decades, annual mortality rate remains high in patients with previous myocardial infarction and left ventricular (LV) dysfunction. A significant proportion of all deaths in patients with chronic ischemic cardiomyopathy is due to pump failure and sudden cardiac death due to malignant ventricular arrhythmias arising from the peri-infarct zone. Although prophylactic implantable cardiac defibrillator (ICD) therapy has been shown to improve survival, prognostic markers for increased risk of death after insertion of ICD are unclear. Based on current guideline selection criteria, up to 35% of all patients underwent appropriate ICD therapies for ventricular arrhythmias by 3-year follow-up. Thus, insights into the mechanisms underlying lethal postinfarct arrhythmias are of major importance and a better risk stratification tool is needed to identify “higher-risk” patients. Recent studies have demonstrated the ability of contrast-enhanced cardiac magnetic resonance to quantify the extent and function of the myocardium in the peri-infarct zone to predict mortality and occurrence of ventricular arrhythmias on follow-up. With advances in echocardiographic deformation imaging techniques such as 2-dimensional speckle tracking, similar site-specific quantifications of regional myocardial function in the infarct, peri-infarct, and remote zones can be rapidly performed semiautomatically. Therefore, the aim of the present prospective, single-blinded, observational study was to identify independent clinical, electrocardiographic (ECG), and echocardiographic determinants of mortality and occurrence of appropriate ICD therapy for ventricular arrhythmias in patients with chronic ischemic cardiomyopathy who received an ICD for primary prevention.
Methods
Patients with chronic ischemic cardiomyopathy were eligible for inclusion in the study if they had previous myocardial infarction >40 days previously, New York Heart Association (NYHA) heart failure functional class ≥II with optimal medical therapy, and LV ejection fraction ≤35%. All patients were referred for ICD implantation for primary prevention as recommended by American College of Cardiology and American Heart Association guidelines. Exclusion criteria included atrial fibrillation, history of surgical ventricular reconstruction, or electrophysiologic ventricular tachycardia ablation.
All patients underwent an extensive baseline history and physical examination, 12-lead electrocardiography, and transthoracic echocardiography before ICD implantation. Baseline clinical variables recorded included NYHA functional class, cardiac risk factors, medications, and glomerular filtration rates (GFRs) calculated by the Modification of Diet in Renal Disease formula as recommended by the National Kidney Foundation, Kidney Disease Outcomes Quality Initiative Guidelines. Baseline ECG variables recorded included heart rate, PR interval, QRS duration, and corrected QT interval calculated by the Bazett formula (corrected QT interval = QT interval/[RR interval] ½ ). Baseline echocardiographic variables recorded included LV volumes, ejection fraction, wall motion score index, and myocardial strain in the infarct, peri-infarct, and remote zones. All baseline clinical and ECG variables were collected by independent observers blinded to echocardiographic results. Similarly, all echocardiographic analyses were performed by separate independent observers blinded to clinical and ECG results. To ensure blinding, clinical/ECG and echocardiographic databases were kept in separate password-protected computers until merging for final data analyses.
All patients were prospectively followed after ICD implantation for occurrence of death and appropriate ICD therapies due to ventricular tachycardia/fibrillation treated with antitachycardia pacing or shocks. From the various clinical, ECG, and echocardiographic variables recorded, independent determinants of all-cause mortality and occurrence of appropriate ICD therapies for ventricular tachycardia/fibrillation were identified.
Transthoracic echocardiography was performed with patients at rest in the left lateral decubitus position and with a commercially available ultrasound transducer and equipment (M4S probe, Vivid 7, GE-Vingmed, Horten, Norway). All transthoracic echocardiographies were performed before ICD insertions and all images were digitally stored on hard disks for off-line analysis (EchoPAC 7.0.0, GE-Vingmed).
A complete 2-dimensional, color, pulse-wave, and continuous-wave Doppler echocardiographic examination was performed. LV end-diastolic volume index and end-systolic volume index were calculated using the Simpson biplane method of disks and corrected for body surface area. LV ejection fraction was calculated and expressed as a percentage.
In the present study, global and segmental longitudinal LV myocardial functions were determined by 2-dimensional speckle tracking strain analyses. To quantify global and segmental LV longitudinal strains, 2-dimensional speckle tracking analyses were performed on standard routine gray-scale images of apical 2-, 3-, and 4-chamber views. During analysis, the endocardial border was manually traced at end-systole and the region-of-interest width adjusted to include the entire myocardium. The software then automatically tracks the myocardium and accepts segments of good tracking quality and rejects poorly tracked segments, and allows the observer to manually over-ride its decisions based on visual assessments of tracking quality. Results of LV longitudinal strain analysis were automatically displayed as a 17-segment polar map model with 17 segmental/regional strain values and a mean global strain value for the entire left ventricle. A previous study had demonstrated a segmental longitudinal strain value >−4.5% as the optimal cut-off value to identify transmural scar tissue on contrast-enhancement cardiac magnetic resonance. Because EchoPAC displays only segmental strain values rounded to whole numbers in the polar map and to increase clinical utility, the optimal longitudinal strain cut-off value to define transmural scar was rounded to the nearest whole number of −5%. Using the 17-segment model, an infarct segment was defined as a longitudinal strain value >−5%. A peri-infarct segment was defined as immediately adjacent to an infarct segment. A remote segment was defined as any segment that was not an infarct or peri-infarct segment. Mean longitudinal strains of the infarct, peri-infarct, and remote zones were then manually averaged ( Figure 1 ).
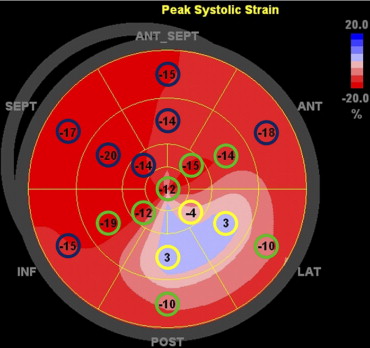
Previous work has reported intra- and interobserver variabilities for longitudinal strain analysis in our laboratory as mean absolute differences ±1 SD of 1.2 ± 0.5% and 0.9 ± 1.0%, respectively.
All deaths were also classified as sudden cardiac death, nonsudden cardiac death, and noncardiac death. Sudden cardiac death was defined as occurring within minutes after onset of acute symptoms, resulting from a documented cardiac arrhythmia, or was not witnessed and occurring unexpectedly without recognizable causes.
ICD therapies were classified as appropriate when they occurred in response to ventricular tachycardia or ventricular fibrillation that was treated with antitachycardia pacing or shocks. These were documented from the ICD device interrogation printouts and confirmed by independent clinical cardiologists blinded to results from the present study. Time to appropriate ICD therapies were recorded from the ICD device interrogation printouts. The primary outcome was death from any cause. The secondary outcome was occurrence of appropriate, successful ICD therapy for ventricular tachycardia/fibrillation.
All continuous variables were not of Gaussian distribution as tested by the Kolmogorov-Smirnov test and were therefore presented as median and interquartile range. Categorical variables were presented as frequencies and percentages and were compared using chi-square test with Yates correction. Mann-Whitney U test was used to compare 2 groups of unpaired continuous variables. Cumulative event rates from time of ICD insertion were calculated using the Kaplan-Meier method and all patients were right censored. All patients who underwent an appropriate ICD therapy (secondary end point) and who subsequently died were also captured in the secondary end point. Log-rank tests for time-to-event data with respect to the primary and secondary outcomes were used for statistical comparison between 2 patient groups dichotomized based on median peri-infarct strain value. Multivariate Cox proportional-hazards models were constructed to identify independent clinical, ECG, and echocardiographic determinants of the primary (all-cause mortality) and secondary (occurrence of appropriate ICD therapies for ventricular tachycardia/fibrillation) outcomes with significant univariate variables (p <0.05) entered as covariates using the stepwise backward likelihood ratio selection method. ICD versus ICD with cardiac resynchronization therapy was also forced into the Cox proportional-hazards models as a factor to determine if there was a difference in all-cause mortality and occurrence of appropriate ICD therapies between the 2 groups. Cox proportional-hazards models were then used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs) for those independent variables. To avoid multicollinearity between univariate predictors, a correlation coefficient <0.7 (corresponding to a tolerance >0.5) was set. A 2-tailed p value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS 16 for Windows (SPSS, Inc., Chicago, Illinois).
Results
Of the 444 patients enrolled in the study, adequate echocardiographic analyses were feasible in 424 patients (95.5%) and constituted the final study population. Median age was 68.5 years (interquartile range 60.6 to 75.3), and 375 patients were men (88.4%). All patients received an ICD for primary prevention. ICD combined with cardiac resynchronization therapy was implanted in 261 patients (61.6%). All patients underwent complete coronary revascularization before ICD implantation.
All baseline clinical, ECG, and echocardiographic variables are presented in Tables 1 and 2 . Most patients (67.7%) were in NYHA functional class III or IV. Median QRS duration was 131 ms (interquartile range 106 to 162) and median LV ejection fraction was 27.0% (interquartile range 21.0 to 32.0).
| Variable | Total Population (n = 424) | Primary Outcome | Secondary Outcome | ||||
|---|---|---|---|---|---|---|---|
| Died (n = 84) | Alive (n = 340) | p Value | ICD Therapy (n = 95) | No ICD Therapy (n = 329) | p Value | ||
| Age (years) | |||||||
| Median | 68.5 | 70.8 | 68.1 | 0.046 | 69.0 | 68.1 | 0.33 |
| Interquartile range | 60.6–75.3 | 63.6–76.4 | 59.9–74.7 | 63.0–75.6 | 60.2–75.0 | ||
| Men | 88.4% | 91.7% | 87.6% | 0.40 | 88.4% | 88.4% | >0.99 |
| Body mass index (kg/m 2 ) | |||||||
| Median | 26.0 | 25.7 | 26.0 | 0.14 | 27.6 | 25.5 | 0.002 |
| Interquartile range | 24.1–28.7 | 23.1–29.0 | 24.4–28.7 | 24.7–29.7 | 24.0–28.4 | ||
| Body surface area (m 2 ) | |||||||
| Median | 1.99 | 1.95 | 1.99 | 0.17 | 2.02 | 1.98 | 0.019 |
| Interquartile range | 1.84–2.11 | 1.80–2.07 | 1.85–2.12 | 1.93–2.14 | 1.83–2.10 | ||
| New York Heart Association heart failure class | 0.024 | >0.99 | |||||
| II | 32.3% | 21.4% | 35.0% | 32.6% | 32.2% | ||
| III and IV | 67.7% | 78.6% | 65.0% | 67.4% | 67.8% | ||
| Hypertension | 32.8% | 34.5% | 32.4% | 0.80 | 36.8% | 31.6% | 0.41 |
| Hyperlipidemia | 69.7% | 69.7% | 69.7% | >0.99 | 64.9% | 71.2% | 0.38 |
| Diabetes mellitus | 27.7% | 40.7% | 24.6% | 0.005 | 20.4% | 29.8% | 0.10 |
| Current smoker | 21.4% | 22.1% | 21.3% | >0.99 | 27.2% | 19.7% | 0.17 |
| Family history ischemic heart disease | 38.3% | 42.3% | 37.3% | 0.49 | 43.7% | 36.7% | 0.29 |
| Systolic blood pressure (mm Hg) | |||||||
| Median | 120 | 115 | 123 | 0.006 | 123 | 120 | 0.84 |
| Interquartile range | 110–140 | 103–130 | 110–140 | 108–140 | 110–138 | ||
| Diastolic blood pressure (mm Hg) | |||||||
| Median | 73 | 70 | 75 | 0.026 | 75 | 71 | 0.09 |
| Interquartile range | 65–81 | 64–80 | 65–83 | 69–83 | 65–80 | ||
| Medication at baseline | |||||||
| Antiplatelets | 48.3% | 39.3% | 50.6% | 0.083 | 42.1% | 50.2% | 0.21 |
| Anticoagulants | 60.7% | 66.7% | 59.2% | 0.26 | 66.3% | 59.0% | 0.25 |
| β Blocker | 65.9% | 64.3% | 66.3% | 0.83 | 64.2% | 66.4% | 0.79 |
| Angiotensin-converting enzyme inhibitor or angiotensin-receptor blocker | 85.5% | 81.0% | 86.7% | 0.24 | 87.4% | 85.0% | 0.68 |
| Calcium channel blocker | 9.2% | 9.5% | 9.2% | >0.99 | 10.5% | 8.9% | 0.77 |
| Sotalol | 9.2% | 7.1% | 9.8% | 0.60 | 11.6% | 8.6% | 0.49 |
| Amiodarone | 16.8% | 22.6% | 15.4% | 0.16 | 14.7% | 17.4% | 0.64 |
| Diuretic | 83.2% | 88.1% | 82.0% | 0.24 | 85.3% | 82.6% | 0.64 |
| Nitrate | 25.8% | 32.1% | 24.3% | 0.18 | 28.4% | 25.1% | 0.60 |
| Statin | 81.5% | 76.2% | 82.8% | 0.21 | 82.1% | 81.3% | 0.99 |
| Device implanted at study entry | |||||||
| Implantable cardiac defibrillator plus cardiac resynchronization therapy | 61.6% | 70.2% | 59.4% | 0.089 | 62.1% | 61.4% | >0.99 |
| Implantable cardiac defibrillator alone | 38.4% | 29.8% | 40.6% | 37.9% | 38.6% | ||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


