The benefit of implantable cardioverter-defibrillators (ICDs) remains controversial in elderly patients and may be attenuated by a greater risk of nonarrhythmic death. We examined the effect of age on outcomes after prophylactic ICD implantation. All patients with coronary artery disease or dilated cardiomyopathy implanted with an ICD for primary prevention of sudden cardiac death in 12 French medical centers were included in a retrospective observational study. The 5,534 ICD recipients were divided according to age: 18 to 59 years (n = 2,139), 60 to 74 years (n = 2,693), and ≥75 years (n = 702). Greater prevalences of coronary artery disease and atrial fibrillation at the time of implant were observed with increasing age (both p <0.0001). During a mean follow-up of 3.1 ± 2.0 years, the annual mortality rate increased with age: 3.1% per year for age 18 to 59 years, 5.7% per year for age 60 to 74 years, and 7.5% per year for age ≥75 years (p <0.001). Older age was independently associated with a greater risk of death (adjusted odds ratio 1.43, 95% confidence interval 1.14 to 1.80 for age 60 to 74 years; and adjusted odds ratio 1.65, 95% confidence interval 1.22 to 2.22 for age >75 years). Proportions of cardiac deaths (55.2%, 57.6%, and 57.0%, p = 0.84), including ICD-unresponsive sudden death (9.9%, 6.0%, and 10.6%, p = 0.08), and rates of appropriate ICD therapies were similar in the 3 age groups. Older age was independently associated with a higher rate of early complications and a lower rate of inappropriate therapies. In conclusion, older patients exhibited higher global mortality after ICD implantation for primary prevention, whereas rates of sudden deaths and of appropriate device therapies were similar across age groups.
Implantable cardioverter-defibrillators (ICDs) in patients at high risk of sudden cardiac death (SCD) have been shown to reduce overall mortality by way of a significant reduction in arrhythmic death. ICD treatment has thus become a standard of care for primary and secondary prevention of SCD in patients with an increased risk of ventricular arrhythmias. Guidelines for prophylactic ICDs are based on randomized controlled trials, which have usually enrolled highly selected patients with few co-morbidities, and only a small number of patients aged >75 years. This contrasts with the average profile of patients with congestive left ventricular systolic dysfunction, who are typically aged >65 years and have multiple co-morbidities. However, because of the aging population, the number of elderly patients being considered for ICD implantation is increasing dramatically. In this specific competing-risk setting, the relative contribution of nonarrhythmic mortality in the elderly may attenuate the benefits of ICD therapy. In the absence of randomized trial data, the benefit of ICD remains controversial in elderly patients. Using data from a large multicenter registry, we examined the effect of age on device-delivered therapies and cause of death after prophylactic ICD implantation in ICD recipients.
Methods
All patients with coronary artery disease or dilated cardiomyopathy implanted with an ICD for primary prevention of SCD at 12 centers (9 university hospitals and 3 private centers) in France from January 2002 through January 2012 were included in this retrospective, observational, single-center study (Défibrillateur Automatique Implantable en Prévention Primaire [DAI-PP]). Patients were implanted in the setting of primary prevention (no previous episode of sudden cardiac arrest or arrhythmic syncope) at the discretion of each investigator. All patients gave their informed consent to participate before ICD implantation. The data file of the DAI-PP study was declared to and authorized by the French data protection committee (Commission Nationale Informatique et Liberté, CNIL, #913203) and is registered on Clinicaltrials.gov ( NCT#01992458 ). The study was conducted in accordance with the principles laid out in the Declaration of Helsinki.
A standardized electronic case report form was used to collect in-hospital and postdischarge data. All baseline variables were acquired at ICD implantation and were defined and categorized according to the published reports or common practice. Age was categorized as ≤59, 60 to 75 and ≥75 years. In addition to gender and New York Heart Association functional status, we collected information on the underlying cause of the heart disease (coronary artery disease or dilated cardiomyopathy). Renal clearance was estimated using the Cockroft–Gault formula and was categorized as ≥60, 30 to 60, and <30 ml/min. QRS duration was categorized as <120, 120 to 150, and ≥150 ms. Left ventricular ejection fraction was collected as a continuous variable and was categorized as ≤30%, 30% to 45%, and >45%. Atrial fibrillation was defined as a history of atrial fibrillation, diagnosed on electrocardiogram or by Holter monitoring, and classified as permanent or paroxysmal. The occurrence of nonsustained ventricular tachycardia recorded on Holter monitoring was also recorded. Information regarding any electrophysiology study was collected (the protocol was left to the discretion of the investigator) and the results were classified as positive or negative. Data on specific co-morbidities and medical history were collected and included cancer, chronic obstructive pulmonary disease, chronic renal failure, chronic liver disease, history of ischemic neurologic attack, and diabetes mellitus. The type of ICD device implanted (biventricular, single chamber, or dual chamber, without the manufacturer’s information), pacemaker functionality, backup pacing rate, programmed detection zones for ventricular and atrial tachyarrhythmias, and programming changes on follow-up were heterogeneous and were conducted at the discretion of the treating cardiologist.
The index date was the date of ICD implantation. Early complications (defined as those that appeared throughout the first 30 days after device implantation) included lead dysfunction, bleeding or hematoma, sepsis, tamponade cardiac, pneumothorax, and death. Complications that occurred after the first month after implant were defined as late complications. After implantation, patients were followed up until the last clinic visit, until heart transplantation, or to the patient’s death. In the French health care system, patients are followed up by the implanting center, and 6-monthly follow-up is recommended. The primary end points for this analysis were the proportion of patients who benefited from at least 1 appropriate ICD therapy during follow-up, and the proportion of overall and cause-specific deaths, according to age groups (18 to 59, 60 to 74, and ≥75 years). End points during follow-up were (1) date of occurrence, type, and number of ICD therapies (appropriate or inappropriate); (2) ICD-related complications; and (3) in the case of death, cause of death. Vital status was obtained from the hospital or the general practitioner and was verified by the National Institute of Statistics Economical Studies. Cause of death was obtained from the investigator and/or by the French Center on medical Causes of Death (CépiDc–INSERM) and was classified as sudden (arrhythmic or not arrhythmic, whenever the assessment was possible); nonsudden cardiovascular (including progressive heart failure, stroke, pulmonary embolism); noncardiovascular; or other specific cause of death (including ICD-related fatal complication). Cause of death was classified as unknown when the quality of the information did not allow the investigators to appropriately identify the cause. Device-interrogation printouts were checked by the local investigator for appropriate and inappropriate ICD therapies. The first appropriate ICD therapy was identified, defined as an episode of ventricular tachycardia/ventricular fibrillation resulting in a single or multiple shocks or/and antitachycardia pacing for arrhythmia termination.
This report was prepared in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement ( http://www.strobe-statement.org/ ). Continuous variables are presented as means (standard deviations) and were compared using the Student t test. Variables that were not normally distributed are described as medians with interquartile ranges and were compared using the Mann–Whitney test. Dichotomous variables are presented as frequencies and percentages and were compared using the chi-square test. A Cox regression analysis was used to identify predictors of death and appropriate therapies, and a binary logistic regression analysis was performed to estimate predictors of inappropriate therapies. p Values <0.05 were considered to indicate statistical significance. All data were analyzed at the Paris Epidemiology Unit of the Cardiovascular Research Center of the French Institute of Health and Medical Research, using SAS program version 9.4 (SAS Institute Inc, Cary, North Carolina).
Results
Of the 5,539 ICD recipients in this registry, age was known in 99.9% (n = 5,534): 38.7% were aged 18 to 59 years, 48.7% 60 to 74 years, and 12.7% ≥75 years ( Table 1 ). The prevalences of coronary artery disease and atrial fibrillation, and the median ejection fraction, increased with higher age group (all p values <0.0001). The prevalence of noncardiovascular co-morbidities (lung disease, liver disease, cancer) also increased with older age. Older patients (≥60 years) were more likely than younger patients to receive a cardiac resynchronization therapy defibrillator device.
| Variable ∗ | Age group (years) | p-value | ||
|---|---|---|---|---|
| 18–59 (n=2139) | 60–74 (n=2693) | ≥75 (n=702) | ||
| Age, mean (Standard deviation) | 51.0 (7.5) | 67.6 (4.4) | 77.8 (2.4) | – |
| Men | 1805 (84.4%) | 2308 (85.7%) | 585 (83.3%) | 0.21 |
| New York Heart Association functional class (n=4557) | ||||
| 1 | 228 (13.1%) | 193 (8.7%) | 60 (9.9%) | <0.0001 |
| 2 | 821 (47.1%) | 843 (38.1%) | 188 (31.1%) | |
| 3 | 631 (36.2%) | 1084 (49.1%) | 334 (55.2%) | |
| 4 | 62 (3.6%) | 90 (4.1%) | 23 (3.8%) | |
| Atrial fibrillation (n=4718) | 266 (14.9%) | 643 (28.0%) | 224 (35.1%) | <0.0001 |
| Coronary artery disease (n=5480) | 1142 (53.9%) | 1701 (63.8%) | 458 (65.9%) | <0.0001 |
| QRS, ms (n=3868) | ||||
| <120 | 582 (40.6%) | 491 (25.9%) | 110 (20.2%) | <0.0001 |
| 120–150 | 452 (31.6%) | 711 (37.6%) | 204 (37.5%) | |
| >150 | 398 (27.8%) | 690 (36.5%) | 230 (42.3%) | |
| Left ventricular ejection fraction (%), median (IQR) (n=5357) | 25 (20-30) | 26 (23-30) | 28 (25-30) | <0.0001 |
| Estimated glomerular filtration rate, mL/min (n=3233) | ||||
| < 30 | 56 (4.4%) | 169 (10.8%) | 53 (13.3%) | <0.0001 |
| 30–60 | 245 (19.4%) | 568 (36.2%) | 186 (46.5%) | |
| >60 | 963 (76.2%) | 832 (53.0%) | 161 (40.3%) | |
| Number of comorbidities ∗ , mean (Standard deviation) | 0.9 (0.7) | 1 (0.8) | 0.9 (0.7) | 0.03 |
| History of stroke | 82 (5.1%) | 104 (5.2%) | 25 (4.4%) | 0.75 |
| Chronic lung disease | 161 (10.1%) | 283 (14.1%) | 70 (12.3%) | 0.001 |
| Liver disease | 39 (2.4%) | 31 (1.5%) | 2 (0.4%) | 0.003 |
| Cancer | 102 (6.4%) | 173 (8.6%) | 39 (6.9%) | 0.03 |
| Diabetes | 68 (26.2%) | 89 (30.8%) | 15 (30.6%) | 0.47 |
| Type of implantable cardioverter defibrillator (n=5486) | ||||
| Single chamber | 670 (31.7%) | 502 (18.8%) | 86 (12.3%) | <0.0001 |
| Dual chamber | 524 (24.8%) | 591 (22.1%) | 163 (23.3%) | |
| Cardiac resynchronization therapy defibrillator | 923 (43.6%) | 1577 (59.1%) | 450 (64.4%) | |
| Treatments | ||||
| Amiodarone | 238 (16.2%) | 492 (25.5%) | 171 (29.7%) | <0.0001 |
| Antiplatelet therapy | 797 (54.3%) | 1128 (58.4%) | 349 (60.7%) | 0.01 |
| ACEi/ARB-II | 1212 (82.6%) | 1590 (82.3%) | 459 (79.8%) | 0.32 |
| Beta-blocker (n=3975) | 1289 (87.8%) | 1620 (83.9%) | 465 (80.9%) | <0.0001 |
| Oral anticoagulation | 433 (29.5%) | 722 (37.4%) | 248 (43.1%) | <0.0001 |
| Sotalol | 7 (0.5 %) | 10 (0.5%) | 4 (0.7%) | 0.83 |
| Spironolactone | 554 (37.7%) | 608 (31.5%) | 120 (20.9%) | <0.0001 |
∗ Percentages calculated on the basis of the total number of known events.
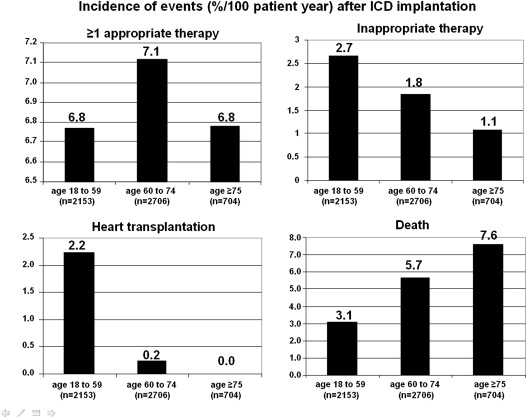
Device-delivered therapies and clinical events were determined at routine clinic visits during a mean follow-up of 3.1 ± 2.0 years ( Table 2 ). A total of 1,180 patients received at least 1 appropriate ICD therapy and 355 received an inappropriate therapy. The risk of early device-related complications increased with older age (p <0.0001).
| Event ∗ | Age group (years) | p-value | ||
|---|---|---|---|---|
| 18–59 (n=2153) | 60–74 (n=2706) | ≥75 (n=704) | ||
| Early device-related complications requiring reintervention | 213 (10.6%) | 376 (14.8%) | 117 (17.4%) | <0.0001 |
| Follow-up available | 2118 (99.0%) | 2659 (98.7%) | 691 (98.4%) | 0.42 |
| Duration, years (standard deviation) | 3.2 (2.3) | 3.1 (2.2) | 2.7 (2.1) | <0.0001 |
| ≥1 appropriate therapy | 466 (22.7%) | 588 (22.8) | 126 (19.4%) | 0.16 |
| Time to first appropriate therapy, years (standard deviation) | 1.2 (1.2) | 1.1 (1.1) | 1.2 (0.9) | 0.89 |
| Late complications | 361 (17.6%) | 395 (15.2%) | 67 (10.3%) | <0.0001 |
| Inappropriate therapy | 182 (8.9%) | 154 (6.0 %) | 19 (2.9%) | <0.0001 |
| Heart transplantation | 155 (7.4%) | 20 (0.8%) | 0 | <0.0001 |
| Death | 212 (10.1%) | 469 (17.9%) | 142 (20.6%) | <0.0001 |
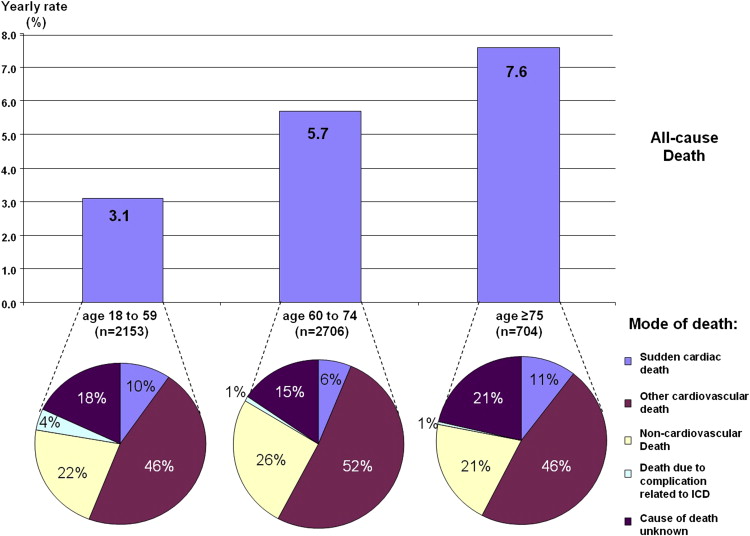
| Cause of death | ||||
| Sudden cardiac death | 21 (9.9%) | 28 (6.0%) | 15 (10.6%) | 0.006 |
| Other cardiovascular death | 96 (45.3%) | 242 (51.6%) | 66 (46.5%) | |
| Non-cardiovascular death | 46 (21.7%) | 122 (26.0%) | 29 (20.4%) | |
| Complication related to implantable cardioverter-defibrillator | 9 (4.3%) | 4 (0.9%) | 1 (0.7%) | |
| Unknown | 40 (18.9%) | 73 (15.6%) | 31 (21.8%) | |
∗ Percentages were calculated on the basis of the total number of known events.
Eight hundred and eleven patients died during 16,693 person-years of follow-up. The all-cause annual mortality rate was 4.9%. Cause-of-death assessment was possible in 679 patients (82.5%). Annual mortality rates increased with age: 3.1% per year for those aged 18 to 59 years, 5.7% per year for patients aged 60 to 74 years, and 7.5% per year for ≥75 years (p <0.001; Figure 1 ). Older age was associated with a greater risk of death (adjusted odds ratio 1.43, 95% confidence interval 1.14 to 1.80 for age 60 to 74 years, and adjusted odds ratio 1.65, 95% confidence interval 1.22 to 2.22 for age >75 years). However, the proportions of cardiac deaths (55.2%, 57.6%, and 57.0%, p = 0.84), including ICD-unresponsive sudden death (9.9%, 6.0%, and 10.6%, p = 0.08), were similar in the 3 age groups ( Figure 2 ). Rates of appropriate ICD therapies were similar in the 3 groups: 7.5% per 100 person-years for patients aged 18 to 59 years, 7.9% for 60 to 74 years, and 7.3% for ≥75 years ( Figure 1 ). Conversely, rates of inappropriate ICD therapies were decreased with older age: 8.9% per 100 person-years for patients aged 18 to 59 years, 6.0% for 60 to 74 years, and 2.9% for ≥75 years, resulting in a lower risk of late complications. In multivariate analysis, older age was independently associated with a greater risk of early complications and all-cause mortality and with a lower risk of inappropriate therapies ( Table 3 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


