There are few reports on early reablation (ER) for early recurrence of atrial fibrillation (AF) after catheter ablation. The present study evaluated the efficacy and significance of ER for early recurrence within a blanking period of 3 months after ablation of both paroxysmal and persistent AF, using a propensity-matched analysis. Of 874 patients who underwent catheter ablation of AF, 389 (45%) had early recurrence. Of these, 78 patients underwent an ER procedure. A total of 132 matched patients (66 in the ER and 66 in the non-ER groups, 82 patients with paroxysmal AF) were included in the analysis. During a mean follow-up of 15.4 months, the patients who underwent ER had a significantly lower recurrence rate than those who did not (29 [44%] vs 42 patients [64%], p = 0.023). The benefit of ER was especially apparent in patients with paroxysmal AF (p = 0.008) but not in those with persistent AF (p = 0.774). However, 24 patients (36%) in the non-ER group did not experience recurrence after a blanking period without any reablation procedure. The total number of reablation sessions was higher in the ER group than in the non-ER group (1.2 ± 0.5 vs 0.4 ± 0.6, p <0.001). Nonetheless, mean number of arrhythmia outpatient clinic visits at follow-up was significantly fewer in the ER group than in the late reablation group. In conclusion, ER for early recurrence of AF after catheter ablation might be effective for preventing recurrence during follow-up, especially for paroxysmal AF.
Although catheter ablation of atrial fibrillation (AF) is a wide-spread treatment in clinical practice, approximately 1/3 of patients experience recurrence of arrhythmia. Moreover, early AF recurrence, mostly within 1 month after catheter ablation, has been reported in 30% to 40% of patients. Early recurrence was considered a transient phenomenon rather than a true recurrence; therefore, a recent expert consensus recommended that early recurrence after ablation should not be classified as a treatment failure. However, several studies reported that early recurrence of AF after an ablation procedure was significantly associated with a later recurrence during follow-up. To treat early recurrence, antiarrhythmic drugs are used during the blanking period after ablation of AF. There are a few reports on early reablation (ER) for early recurrence during the blanking period after the first ablation. Because previous studies were conducted with no control of baseline characteristics, or focused on paroxysmal AF alone, the independent effect of ER when used to treat early recurrence of all types of AF is unclear. Thus, the present study sought to examine the significance of ER for early recurrence within a blanking period of 3 months after ablation of both paroxysmal and persistent AF, using a propensity score–matched analysis.
Methods
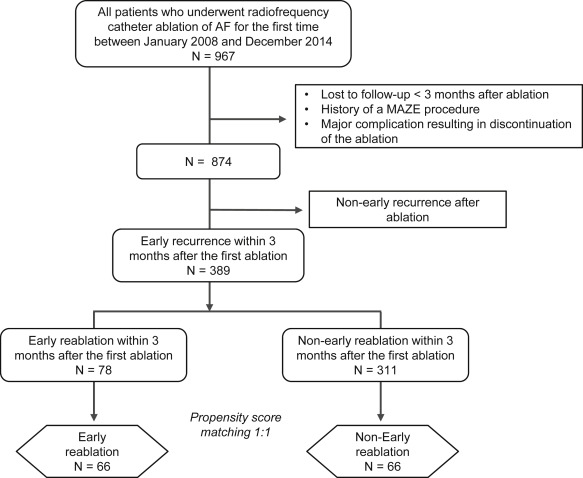
The study population was recruited retrospectively from a catheter ablation database at the Nagoya University Hospital. This ablation database was approved by our institutional ethics committee. Patients who underwent radiofrequency catheter ablation of AF for the first time from January 2008 to December 2014 were initially extracted for the study (n = 967). We excluded those patients who met the following exclusion criteria: (1) loss to follow-up within 3 months after catheter ablation, (2) a history of a maze procedure, and (3) development of a major complication resulting in discontinuation of the ablation procedure. Of the remaining 874 patients, 389 patients (45%) with early recurrence within 3 months after the first catheter ablation of AF were evaluated. In the early recurrence group, 78 patients underwent an ER procedure and 311 patients did not undergo the ER procedure. Then, we assessed the outcomes of those who underwent ER within 3 months of the first ablation and compared them with those of a cohort of similar patients who did not undergo ER using a propensity score–matched analysis ( Figure 1 ). The time trends of early recurrence and ER rates from 2008 to 2014 are shown as Supplementary Figure 1 . The indications for catheter ablation of AF complied with the latest guidelines. Before the procedure, informed consent was obtained from all patients, in accordance with our hospital guidelines.
Patients who were scheduled for catheter ablation were admitted 1 day before the procedure. At admission, baseline blood testing, echocardiography, electrocardiography, and Holter examination were performed. Antiarrhythmic agents were stopped 5 half-lives before ablation, except for amiodarone and bepridil that were stopped over 1 week before the procedure. Transesophageal echocardiography was performed in all patients to confirm the absence of an atrial thrombus. Anticoagulant drugs were continued during the procedure.
In the ablation procedure, vascular access was obtained through the right and left femoral and left subclavian veins. After transseptal puncture under intracardiac echocardiography monitoring, two 8-Fr sheaths and an 8.5-Fr steerable sheath were introduced into the left atrium. Then, using a circular mapping catheter (Lasso; Biosense Webster Inc., Diamond Bar, California) placed on the ostium of each pulmonary vein (PV), encircling PV isolation was performed with a 3.5-mm tip, open-irrigated ablation catheter (Biosense Webster Inc.) to achieve electric isolation of the PV potential. All ablation procedures were performed using a 3-dimensional electroanatomical mapping system (CARTO; Biosense Webster Inc.). For the most part, paroxysmal AF and early persistent AF were treated by PV isolation and cavotricuspid isthmus ablation alone. However, in patients with prolonged persistent AF, atrial tachycardia, or evidence of non-PV foci, additional linear ablation, superior vena cava isolation, and complex fractionated atrial electrogram ablation were performed. After the PV isolation, a waiting observation period of at least 10 minutes was provided to check for acute reconnection.
Patients remained hospitalized under continuous rhythm monitoring for 3 days after the procedure. After the discharge, patients were followed up through the outpatient clinic at minimum every month after ablation. At the time of each follow-up visit, patients underwent 12-lead electrocardiography and were asked about any symptoms related to the presence of arrhythmia. If patients were suspected to have emergent arrhythmia, additional Holter monitoring and a short-duration follow-up were performed. If the patients noticed any rhythm disorder during the follow-up, they were recommended to make a telephone call to arrange an early visit to the hospital and an electrocardiographic examination. Twenty-four–hour Holter monitoring was performed in all patients 1 month after ablation. The antiarrhythmic drugs were systematically discontinued after the first ablation. If the patients had an episode of AF, antiarrhythmic drugs that had been discontinued before the procedure were re-administered.
Early recurrence was defined as any recurrence of AF or atrial tachycardia lasting for at least 30 seconds during the blanking period of 3 months after the first ablation. Reablation during the blanking period was defined as ER. Even if the patients underwent ER, the blanking period remained the same, with reference to the first-time procedure. Recurrence at follow-up was any AF or atrial tachycardia of >30 seconds in duration. Discontinuation of antiarrhythmic agents was decided on the basis of freedom from recurrence of any atrial arrhythmia for >3 to 6 months after ablation.
AF was classified as either paroxysmal (spontaneously terminated and lasting ≤7 days) or persistent (sustained and lasting >7 days). This retrospective study was performed in accordance with the Declaration of Helsinki. The patients’ baseline characteristics, co-morbidities, and therapeutic details were obtained from hospital medical records.
Comparison of the differences in the baseline characteristics were analyzed using Student’s t test for parametric data and Mann-Whitney U tests for nonparametric data. Categorical variables were compared using the chi-square test or Fisher’s exact test. The Kaplan-Meier method was used to estimate event-free survival, and the differences between the curves were compared using the log-rank test. To minimize differences and overcome the bias in the baseline characteristics resulting from the study design, we constructed a propensity score model for ER or non-ER among the early recurrence group. We calculated the propensity score using a multivariable logistic regression model using ER as the dependent variable and including the following baseline factors: age, male sex, body mass index, paroxysmal AF, duration of AF, symptoms before ablation and at early recurrence, antiarrhythmic medication, co-morbidities, device implantation, left atrial diameter, left ventricular ejection fraction, laboratory data, and ablation procedures. Then, 1:1 nearest-neighbor greedy matching was performed. The outcomes and measured covariates were compared between groups using the paired t test for continuous variables and McNemar’s test for categorical data. A p value <0.05 was considered statistically significant.
Results
A total of 132 matched patients (66 in the ER group and 66 in the non-ER group) were included. Clinical variables were well balanced between the ER and non-ER groups in the matched cohort ( Table 1 ). During a mean follow-up of 15.4 months, the patients who underwent ER had a significantly lower recurrence rate than those who did not (29 [44%] vs 42 patients [64%], p = 0.023). In the non-ER group, 24 patients (36%) did not experience recurrence without additional reablation. The Kaplan-Meier survival curves for freedom from AF recurrence after the blanking period showed that the ER group had a significant better prognosis than the non-ER group (p = 0.018; Figure 2 ). The total number of reablations after the first ablation, including ER, was significantly higher in the ER group than in the non-ER group (1.2 ± 0.5 vs 0.4 ± 0.6, p <0.001).
| Parameter | Overall Population (n = 389) | p-Value | Propensity Score Matched (n = 132) | p-Value | ||
|---|---|---|---|---|---|---|
| Early Reablation (n = 78) | Non-Early Reablation (n = 311) | Early Reablation (n = 66) | Non-Early Reablation (n = 66) | |||
| Age (years) | 64.9 ± 9.4 | 61.7 ± 11.3 | 0.025 | 63.7 ± 9.4 | 64.9 ± 10.9 | 0.532 |
| Men | 57 (73%) | 231 (74%) | 0.829 | 48 (73%) | 44 (67%) | 0.449 |
| Body mass index (kg/m) | 23.5 ± 3.1 | 24.9 ± 9.3 | 0.203 | 23.9 ± 2.9 | 23.9 ± 3.5 | 0.985 |
| Type of atrial fibrillation | ||||||
| Paroxysmal | 50 (64%) | 184 (59%) | 0.426 | 41 (62%) | 41 (62%) | 0.999 |
| Persistent | 28 (36%) | 127 (41%) | 0.426 | 25 (38%) | 25 (38%) | 0.999 |
| Duration of atrial fibrillation (years) | 2.0 (0.5–6.3) | 2.0 (0.5–6.0) | 0.974 | 2.0 (0.5–6.0) | 1.7 (0.4–6.0) | 0.453 |
| Symptoms before ablation | 54 (69%) | 179 (58%) | 0.060 | 44 (67%) | 41 (62%) | 0.586 |
| Medication | ||||||
| Class I | 36 (46%) | 107 (35%) | 0.054 | 31 (47%) | 27 (41%) | 0.483 |
| Class III | 11 (14%) | 44 (14%) | 0.992 | 8 (12%) | 11 (17%) | 0.457 |
| Amiodarone | 3 (4%) | 14 (5%) | 0.800 | 3 (5%) | 3 (5%) | 0.999 |
| None | 31 (40%) | 160 (51%) | 0.065 | 27 (41%) | 28 (42%) | 0.860 |
| Number of antiarrhythmic drugs | 0.8 ± 0.8 | 0.8 ± 0.9 | 0.723 | 0.8 ± 0.7 | 0.9 ± 0.9 | 0.356 |
| Comorbidities | ||||||
| Hypertension | 45 (58%) | 138 (44%) | 0.035 | 35 (53%) | 33 (50%) | 0.728 |
| Diabetes mellitus | 13 (17%) | 59 (19%) | 0.639 | 12 (18%) | 12 (18%) | 0.999 |
| Heart failure | 11 (14%) | 40 (13%) | 0.772 | 10 (15%) | 9 (14%) | 0.804 |
| Coronary artery disease | 7 (9%) | 23 (7%) | 0.640 | 5 (8%) | 6 (9%) | 0.753 |
| Stroke or transient ischemic attack | 9 (12%) | 40 (13%) | 0.753 | 8 (12%) | 6 (9%) | 0.572 |
| Previous device implantation | 2 (3%) | 18 (6%) | 0.389 | 2 (3%) | 3 (5%) | 0.999 |
| Echocardiographic data | ||||||
| Left atrial diameter (mm) | 40.6 ± 6.2 | 40.4 ± 6.9 | 0.845 | 40.8 ± 6.6 | 41.6 ± 5.8 | 0.508 |
| Left ventricular ejection fraction (%) | 59.2 ± 9.7 | 59.4 ± 10.5 | 0.875 | 58.9 ± 10.1 | 58.3 ± 11.7 | 0.744 |
| CHADS2 score | 1.3 ± 1.2 | 1.2 ± 1.1 | 0.380 | 1.2 ± 1.2 | 1.2 ± 1.1 | 0.940 |
| CHA2DS2-VASc score | 2.2 ± 1.6 | 1.9 ± 1.6 | 0.131 | 2.1 ± 1.6 | 2.2 ± 1.7 | 0.709 |
| Laboratory data | ||||||
| Hs-CRP (mg/L) | 0.60 (0.30–1.70) | 0.70 (0.30–1.40) | 0.774 | 0.50 (0.30–1.60) | 0.80 (0.30–1.40) | 0.249 |
| eGFR (mL/min/1.73m 2 ) | 67.6 ± 17.8 | 70.1 ± 19.3 | 0.302 | 67.9 ± 15.4 | 66.2 ± 20.0 | 0.577 |
| B-type natriuretic peptide levels (pg/dL) | 75.5 (38.0–145.8) | 57.2 (28.0–127.1) | 0.139 | 73.5 (35.2–145.8) | 80.9 (38.7–152.5) | 0.598 |
| Ablation procedure | ||||||
| Pulmonary vein isolation | 78 (100%) | 311 (100%) | N/A | 66 (100%) | 66 (100%) | N/A |
| Cavotricuspid isthmus | 72 (92%) | 255 (82%) | 0.026 | 60 (91%) | 61 (92%) | 0.753 |
| Left atrial linear ablation | 21 (27%) | 123 (40%) | 0.039 | 21 (32%) | 23 (35%) | 0.712 |
| Mitral isthmus line | 17 (22%) | 89 (29%) | 0.226 | 17 (26%) | 20 (30%) | 0.561 |
| Complex fractionated atrial electrogram | 13 (17%) | 73 (24%) | 0.195 | 13 (20%) | 16 (24%) | 0.528 |
| Superior vena cava isolation | 6 (8%) | 35 (11%) | 0.360 | 6 (9%) | 5 (8%) | 0.753 |
| At the early recurrence | ||||||
| Recurrence type | ||||||
| Atrial fibrillation | 58 (74%) | 230 (74%) | 0.942 | 48 (73%) | 48 (73%) | 0.999 |
| Atrial tachycardia or atrial flutter | 20 (26%) | 81 (26%) | 0.942 | 18 (27%) | 18 (27%) | 0.999 |
| Symptoms | 56 (72%) | 166 (53%) | 0.003 | 45 (68%) | 44 (67%) | 0.853 |
| Early recurrence days | 3.0 (2.0–28.0) | 3.0 (2.0–18.8) | 0.391 | 3.0 (2.0–31.0) | 2.0 (2.0–11.5) | 0.260 |
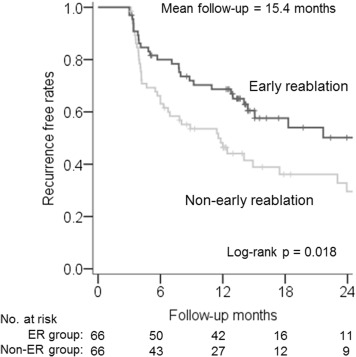
Table 2 gives a comparison of the procedure outcomes of the second ablation between the ER group (n = 66) and patients in the non-ER group who underwent late reablation (n = 24). There were no significant differences in the prevalence of PV reconnection, number of PV reconnections, or additional ablation procedures between the 2 groups. Major complications occurred during the reablation procedure in 2 patients in the ER group and in 1 in the late reablation group. Specifically, these complications were a pericardial effusion requiring drainage and cerebral infarction in the ER group and pericardial effusion requiring drainage in the late reablation group. These complications occurred at the post-ablation procedures, and all reablation procedures were successfully performed.
| Parameter | Early Reablation (n = 66) | Non-Early Reablation (n = 66) | p-Value |
|---|---|---|---|
| Recurrence | 29 (44%) | 42 (64%) | 0.023 |
| Recurrence days after blanking period (days) | 305 ± 279 | 295 ± 368 | 0.892 |
| Medication at last follow-up | |||
| Anti-arrhythmic drugs | 32 (48%) | 36 (54%) | 0.892 |
| Class I | 22 (33%) | 20 (30%) | 0.709 |
| Class III | 10 (15%) | 16 (24%) | 0.189 |
| None | 34 (52%) | 30 (46%) | 0.892 |
| Total number of reablation including blanking period | 1.2 ± 0.5 | 0.4 ± 0.6 | <0.001 |
| Reablation after blanking period | 14 (21%) | 24 (38%) | 0.055 |
| Reablation days after first procedure (days) | 58 ± 23 | 554 ± 640 ∗ | <0.001 |
| Procedure outcomes during the second ablation | |||
| Pulmonary vein reconnection | 51 (77%) | 20 (83%) ∗ | 0.533 |
| Left superior pulmonary vein | 31 (47%) | 14 (58%) ∗ | 0.340 |
| Left inferior pulmonary vein | 22 (33%) | 10 (42%) ∗ | 0.465 |
| Right superior pulmonary vein | 41 (62%) | 15 (63%) ∗ | 0.974 |
| Right inferior pulmonary vein | 39 (59%) | 10 (42%) ∗ | 0.142 |
| Number of pulmonary vein reconnection | 2.0 ± 1.4 | 2.0 ± 1.3 ∗ | 0.963 |
| Additional ablation procedures | |||
| Cavotricuspid isthmus | 20 (30%) | 5 (21%) ∗ | 0.375 |
| Left atrial linear ablation | 14 (21%) | 7 (29%) ∗ | 0.430 |
| Mitral isthmus line | 27 (41%) | 14 (58%) ∗ | 0.142 |
| Complex fractionated atrial electrogram | 9 (14%) | 4 (17%) ∗ | 0.740 |
| Superior vena cava isolation | 24 (36%) | 4 (17%) ∗ | 0.074 |
| Major complications at reablation | 2 (3%) | 1 (4%) ∗ | 0.791 |
∗ Data are presented in 24 patients who underwent late reablation in the non-early reablation group.
The patients were divided into a paroxysmal AF group (82 patients) and a persistent AF group (50 patients), and the ER and non-ER groups were compared in each of these groups. No significant difference regarding baseline characteristics, examination data, and ablation procedures was observed between the ER and non-ER groups, both in the paroxysmal and persistent AF groups ( Table 3 ). Figure 3 shows the distribution of the time to early recurrence after ablation among the paroxysmal and persistent AF groups. The patients with paroxysmal AF were more likely to experience early recurrence within 7 days after ablation compared with those with persistent AF. After the blanking period, there was a significant difference in recurrence rate between the ER and non-ER groups in patients with paroxysmal AF (15 [37%] vs 27 patients [66%], p = 0.008). In contrast, no significant benefit of ER was found in patients with persistent AF (14 [56%] vs 15 patients [60%], p = 0.774). Figure 4 shows that the ER group had a better prognosis than the non-ER group among patients with paroxysmal AF (p = 0.005) but not in those with persistent AF (p = 0.856).
| Parameter | Paroxysmal Atrial Fibrillation (n = 82) | p-Value | Persistent Atrial Fibrillation (n = 50) | p-Value | ||
|---|---|---|---|---|---|---|
| Early Reablation (n = 41) | Non-Early Reablation (n = 41) | Early Reablation (n = 25) | Non-Early Reablation (n = 25) | |||
| Age (years) | 65.3 ± 8.2 | 68.5 ± 8.4 | 0.087 | 61.2 ± 10.7 | 58.9 ± 11.9 | 0.481 |
| Men | 30 (73%) | 23 (56%) | 0.106 | 18 (72%) | 21 (84%) | 0.306 |
| Body mass index (kg/m) | 23.9 ± 3.2 | 23.7 ± 3.5 | 0.770 | 23.8 ± 2.5 | 24.2 ± 3.4 | 0.656 |
| Duration of atrial fibrillation (years) | 1.2 (0.4–4.0) | 2.0 (0.6–4.8) | 0.830 | 4.5 (1.1–8.4) | 3.5 (0.6–10.0) | 0.210 |
| Symptoms before ablation | 34 (83%) | 33 (81%) | 0.775 | 10 (40%) | 8 (32%) | 0.556 |
| Medication | ||||||
| Class I | 28 (68%) | 22 (54%) | 0.174 | 3 (12%) | 5 (20%) | 0.702 |
| Class III | 3 (7%) | 6 (15%) | 0.319 | 5 (20%) | 5 (20%) | 0.999 |
| Amiodarone | 1 (2%) | 2 (5%) | 0.999 | 2 (8%) | 1 (4%) | 0.999 |
| None | 10 (24%) | 13 (32%) | 0.624 | 17 (68%) | 15 (60%) | 0.556 |
| Number of antiarrhythmic drugs | 1.0 ± 0.7 | 1.2 ± 1.0 | 0.380 | 0.4 ± 0.6 | 0.4 ± 0.6 | 0.626 |
| Comorbidities | ||||||
| Hypertension | 23 (56%) | 25 (61%) | 0.654 | 12 (48%) | 8 (32%) | 0.248 |
| Diabetes mellitus | 8 (20%) | 8 (20%) | 0.999 | 4 (16%) | 4 (16%) | 0.999 |
| Heart failure | 4 (10%) | 4 (10%) | 0.999 | 6 (24%) | 5 (20%) | 0.733 |
| Coronary artery disease | 3 (7%) | 4 (10%) | 0.999 | 2 (8%) | 2 (8%) | 0.999 |
| Stroke or transient ischemic attack | 4 (10%) | 6 (15%) | 0.500 | 4 (16%) | 0 (0%) | 0.110 |
| Previous device implantation | 2 (5%) | 2 (5%) | 0.999 | 0 (0%) | 1 (4%) | 0.999 |
| Echocardiographic data | ||||||
| Left atrial diameter (mm) | 39.6 ± 6.3 | 40.9 ± 6.8 | 0.347 | 43.1 ± 6.5 | 42.7 ± 3.6 | 0.809 |
| Left ventricular ejection fraction (%) | 61.2 ± 10.3 | 59.7 ± 12.5 | 0.556 | 55.1 ± 8.7 | 55.9 ± 10.0 | 0.760 |
| CHADS2 score | 1.2 ± 1.1 | 1.5 ± 1.1 | 0.204 | 1.3 ± 1.4 | 0.8 ± 0.8 | 0.090 |
| CHA2DS2-VASc score | 2.2 ± 1.4 | 2.8 ± 1.6 | 0.073 | 2.1 ± 1.9 | 1.4 ± 1.3 | 0.128 |
| Laboratory data | ||||||
| Hs-CRP (mg/L) | 0.40 (0.20–1.50) | 0.80 (0.30–1.40) | 0.050 | 0.80 (0.40–2.10) | 0.70 (0.30–1.70) | 0.621 |
| eGFR (mL/min/1.73m 2 ) | 66.6 ± 12.3 | 65.6 ± 20.2 | 0.798 | 70.2 ± 19.5 | 67.1 ± 20.1 | 0.592 |
| B-type natriuretic peptide levels (pg/dL) | 60.6 (25.1–115.1) | 86.6 (34.6–155.3) | 0.118 | 87.1 (57.3–237.9) | 66.7 (44.1–148.8) | 0.204 |
| Ablation procedure | ||||||
| Pulmonary vein isolation | 41 (100%) | 41 (100%) | N/A | 25 (50%) | 25 (100%) | N/A |
| Cavotricuspid isthmus | 37 (90%) | 36 (88%) | 0.999 | 23 (92%) | 25 (100%) | 0.490 |
| Left atrial linear ablation | 4 (10%) | 3 (7%) | 0.999 | 17 (80%) | 20 (80%) | 0.333 |
| Mitral isthmus line | 4 (10%) | 4 (10%) | 0.999 | 13 (52%) | 16 (64%) | 0.390 |
| Complex fractionated atrial electrogram | 2 (5%) | 4 (10%) | 0.675 | 11 (44%) | 12 (48%) | 0.777 |
| Superior vena cava isolation | 0 (0%) | 0 (0%) | 0.999 | 6 (24%) | 5 (20%) | 0.733 |
| At the early recurrence | ||||||
| Recurrence type | ||||||
| Atrial fibrillation | 30 (73%) | 33 (80%) | 0.432 | 18 (72%) | 15 (60%) | 0.370 |
| Atrial tachycardia or atrial flutter | 11 (27%) | 8 (20%) | 0.432 | 7 (28%) | 10 (40%) | 0.370 |
| Symptoms | 33 (81%) | 32 (78%) | 0.785 | 12 (48%) | 12 (48%) | 0.999 |
| Early recurrence days | 3.0 (2.0–24.5) | 2.0 (2.0–5.5) | 0.323 | 3.0 (2.0–32.0) | 3.0 (2.0–21.0) | 0.470 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


