Fig. 24.1
CO2 removal at different levels of sweep gas flow (SGF) during awake vvECMO. Increasing levels of SGF were associated with an increase of extracorporeal CO2 removal and a simultaneous decrease of the CO2 removed by the lungs. The decrease in total CO2 removal observed between 1 and 3 l/min of SGF resulted from a decrease in oxygen consumption (and metabolic CO2 production) due to the drop of a high work of breathing. At an SGF of 8 l/min, the metabolic CO2 production was nearly totally removed by the artificial lung
The awake vaECMO approach has been used when lung failure was associated with severe pulmonary hypertension and right heart failure. In a recent report of vaECMO as a bridge to recovery or to heart transplantation for primary cardiogenic shock or cardiac arrest [13], the treatment protocol recommended awake ECMO, if possible, especially when a need for prolonged extracorporeal support was foreseen. In this report of 16 patients with a 50 % survival at hospital discharge, 29 % of 3,514 h on ECMO was spent as awake ECMO, and 4 patients were never intubated. This report shows that a strategy of awake ECMO, with the purpose of reducing the risk of infection and with the advantage of easy and prompt neurologic assessment, is feasible in several patients with cardiogenic shock.
Finally, in cases of severe chronic pulmonary hypertension, a bridge to lung transplantation has been obtained by surgical central implantation of a Novalung® ILA® in parallel to the pulmonary circulation, between the pulmonary artery and either the left atrium [14] or a pulmonary vein [15]. This pumpless approach, denoted as “paracorporeal artificial lung” (PAL), is made possible by the combination of a powerful hypertrophic right ventricle with the low resistance typical of the ILA® oxygenator. Once stabilized after PAL implantation, patients were extubated and maintained awake up to lung transplantation [14, 15].
24.5 Cannulation Sites
The choice about cannulation sites partly depends on whether or not the clinical staff is oriented to implement a program of active physical therapy including patient ambulation. If this is the case, the femoral vessels cannot be used, and vvECMO has been commonly applied through the right internal jugular vein with a bi-caval double-lumen Avalon Elite® cannula [8, 12, 16–18]. In patients needing a substantial oxygenation support and hence a high blood flow, a very large cannula (27–31 F) must be implanted, but its prolonged use is frequently complicated by deep vein thrombosis of the upper extremities [19]. The cannula can be stabilized by tunnelization of the right jugular access [12] or by using a left subclavian access [20]. An upper-extremity approach suitable for ambulation has also been used in one case of vaECMO, by using the left axillary vein and artery [21].
The alternative approach with two femoral cannulas, although not compatible with ambulation, does not impede active physical therapy in bed, even including the lower extremities. Usually total leg immobility is unnecessary, and the long wire-reinforced cannulas used for femorofemoral ECMO do not kink with moderate thigh flexion. In our practice, we recommend leg rest only in case of bleeding through the cannula insertion points. The bi-femoral approach is used for both vvECMO and vaECMO and is very practical in patients who need CPAP to improve oxygenation; the free neck allows application of helmet CPAP [22], which is much better tolerated than masks and is the perfect interface for a prolonged treatment.
Whether or not the awake patient on ECMO should also be enabled to ambulate, it is questionable. Walking while on ECMO may expose the patient to additional risks and requires assistance by extra personnel. In a recent Italian report of ECMO as bridge to lung transplantation, patients bridged with awake ECMO had much lower morbidity and an easier clinical course, with less need of postoperative IMV, shorter posttransplant length of stay both in ICU and in hospital, and a lower incidence of critically ill polyneuropathy/myopathy than intubated patients [7]. In this group of patients, awake ECMO was always applied with femoral cannulation; therefore, it can be inferred that the advantages of awake ECMO are maintained, at least partly, even if the ambulation option is excluded.
As already mentioned, the femoral approach is the most practical way to apply a pumpless av bypass, while the PAL in parallel to the pulmonary circulation obviously requires a central cannulation.
24.6 Management of Awake ECMO
24.6.1 Setup and Start
Power and stability of the extracorporeal system are the two keystones for effectiveness and good tolerance of the treatment, and this is particularly important in awake patients, who have the ability to react to the changes in the artificial lung function.
The amount of extracorporeal CO2 removal can be easily regulated by changing the sweep gas flow of the artificial lung (Fig. 24.1). Extracorporeal CO2 removal and oxygen transfer decrease patient’s need of alveolar ventilation and hence respiratory drive and work of breathing [9]. If ECMO is working properly, shortly after start, the patient feels much better, perceiving the function of the implanted artificial lung like an apparent rapid improvement of the impaired function of his/her native lung. In turn, in case of drop of extracorporeal support performance due to technical problems, the patient will suddenly feel and react like suffocating.
In order to achieve a favorable interaction, it is important to choose the extracorporeal support according to the failing function of the native lung. If the oxygenation impairment is very severe, it is fundamental to implant large-size cannulas, in order to allow the setting of a high extracorporeal blood flow with good stability and without excessive negative/positive pressures. When CO2 elimination is the major problem, especially if lung compliance is very low, it is important to set an adequately high ventilation of the artificial lung in order to deeply depress the respiratory drive. The resulting reduction of a previously extremely high work of breathing will involve a decrease of the patient’s oxygen consumption, thus rising venous oxygen saturation and hence contributing to improve arterial oxygenation. Moreover, the reduction of metabolic CO2 production (Fig. 24.1) will further contribute to unload the patient.
However, unless the patient is breathing pure oxygen with adequate CPAP, excessive CO2 removal with vvECMO may result in oxygen desaturation due to alveolar hypoventilation and derecruitment, as a consequence of excessive depression of the respiratory drive. In the clinical case of Fig. 24.2, this is what happened on day 1 with a gas flow of 6 l/min and on day 7 with a gas flow of 8 l/min (corresponding to an extracorporeal CO2 removal of 192 and 208 ml/min, respectively).
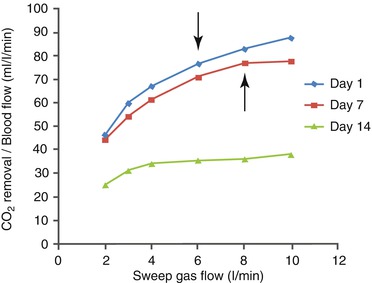

Fig. 24.2
Relationship between sweep gas flow (SGF) and extracorporeal CO2 removal (expressed as ml/min per liter of blood flow) in a clinical case of awake vvECMO. Blood flow on days 1, 7, and 14 was 2.5, 2.7, and 4.2 l/min, respectively. Compared to day 1, the CO2 removal function slightly deteriorated on day 7, while it greatly deteriorated on day 14. Oxygen desaturation (values not showed) associated with critical alveolar hypoventilation due to excessive extracorporeal CO2 removal occurred at an SGF of 6 l/min on day 1 and 8 l/min on day 7 (arrows); it never occurred on day 14, due to the limited CO2 removal even at high SGF
In some patients bridged to lung transplantation for advanced lung fibrosis, lung compliance can be so low that every breath is associated with deep inspiratory breathing efforts: in these cases, extracorporeal CO2 removal can be virtually complete, and the patient will remain nearly apneic in an atmosphere of pure oxygen obtained by a CPAP helmet; his/her will breathe just when his/her needs to speak or to cough.
24.6.2 Adaptation to Changes in Extracorporeal Support Function
Once a good compensation of the failing respiratory function is achieved, the system may become unstable because of an imbalance between the extracorporeal support and the native respiratory function.
Thanks to its pump, vvECMO is typically more stable than an av pumpless respiratory support, during which the extracorporeal blood flow (that affects CO2 removal, to a given extent) strongly depends on arterial blood pressure [11]. With an av pumpless support, blood flow and CO2 removal can also be greatly affected by movements of the lower extremities, with increased resistance of the cannulas due to bending and position changes.
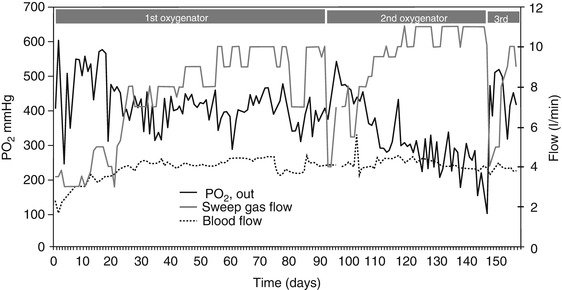
Although much more rarely, also during vvECMO blood flow can become unstable, due to bending of cannulas or tubes or to poor blood drainage because of hypovolemia. However, when cannulation and volemic status are adequate, the main technical factor requiring an adjustment of ECMO settings is represented by the progressive deterioration of the artificial lung function. In Fig. 24.2 it can be noted how, at any level of sweep gas flow, the CO2 removal efficiency was much lower on day 14 than on ECMO start or on day 7; moreover, on day 14 no further increase of CO2 removal could be obtained by increasing the gas flow above 4 l/min, indicating the need for oxygenator replacement. The progressive deterioration of the artificial lung may affect first CO2 removal and only later oxygenation (Fig. 24.3). Up to a given extent, the deterioration of the extracorporeal CO2 removal function can be compensated by increasing the ventilation of the artificial lung. Manual adjustment of ECMO gas flow to compensate for progressive deterioration is particularly important in the awake patient, who otherwise would react, thus becoming unstable.