INTERMACS profile
Short description
1
Critical cardiogenic shock
2
Progressive decline on inotropes
3
Stable, but inotrope dependent
4
Symptoms at rest; home on oral therapy
5
Exertion intolerant
6
Exertion limited
7
Advanced NYHA class III symptoms
Not surprisingly, preoperative severity of cardiac decompensation correlates with outcomes following MCS. INTERMACS profile levels 1 and 2 have the poorest survival – approximately 5–8 % lower. Overall, actuarial survival is 80 and 70 % at 1 and 2 years, respectively [2]. With recognition of suboptimal outcomes for profile level 1 patients, combined with improving technology, increasingly earlier VAD implantation is being performed. At present, INTERMACS level 1 patients account for 16.6 % of new implants [2].
It is anticipated that as more devices receive approval and wider adoption, the ability to track trends in MCS will provide further insights and guide future care. Efforts are underway to consolidate data into a single registry – the International Society for Heart & Lung Transplantation (ISHLT) Mechanical Assisted Circulatory Support (IMACS) Registry [4].
19.3 Intra-aortic Balloon Pump (IABP) Counterpulsation Therapy
IABP therapy has been employed since Kantrowitz’s initial publication in 1968 [5]. The success, combined with relative speed of insertion and simplicity of the IABP, has led to it becoming the most widely used initial assist device.
The IABP is a helium-filled balloon affixed to the tip of a catheter, generally inserted retrograde through the femoral artery. The tip of the balloon is optimally positioned 1–2 cm distal to the origin of the left subclavian artery. The IABP operates on a volume-displacement counterpulsation principle to exert its hemodynamic effect. Thus, the balloon inflates in diastole and deflates in systole.
Physiologically, the IABP augments coronary perfusion, reduces left ventricular afterload, and reduces left ventricular wall tension. Diastolic inflation increases coronary perfusion pressure and coronary blood (and hence oxygen) supply. Increased diastolic pressure may improve coronary collateral perfusion, as well as systemic perfusion.
Systolic deflation just prior to isovolumetric contraction results in afterload reduction, reduced LV wall tension, increased stroke volume, and cardiac output augmentation. Management with IABP requires appropriate timing to adjust inflation and deflation, guided by the arterial pressure waveform. Modern devices are substantially automated, simplifying monitoring and timing.
Indications for IABP include cardiogenic shock, coronary ischemia, and dysrhythmias. Complications post-myocardial infarction can also supported, such as ventricular septal defects (VSDs), acute severe mitral regurgitation secondary to papillary muscle rupture, and left ventricular aneurysms.
IABP use is absolutely contraindicated in aortic insufficiency and aortic dissection. Caution must be advised in patients with severe atherosclerotic disease, significant peripheral vascular disease, abdominal aortic aneurysm, and graft replacement of the iliac or femoral arteries.
For patients in extremis, IABP may be insufficient to support severe dysrhythmias or the degree of hemodynamic embarrassment. Additionally, immobilization of the patient, combined with peripheral arterial access, limit the effective duration of support. Hemolysis and platelet consumption may also occur.
The hemodynamic benefits of IABP support have been reported to improve survival in cardiogenic shock following acute myocardial infarction (MI) [6]. The joint American College of Cardiology (ACC) and American Heart Association (AHA) guidelines have assigned IABP a class IIa recommendation as a management option in this scenario [7]. European guidelines suggest consideration of IABP in this situation as a class IIb recommendation [8]. Previous international guidelines had supported IABP usage as a class I indication for post-MI shock, but recently IABP efficacy has been questioned in the IABP-SHOCK II trial [9, 10]. Nevertheless, previous widespread adoption and clinician comfort with IABP mean it currently remains a first-line management tool for hemodynamic support.
19.4 Percutaneous Mechanical Circulatory Support
IABP limitations, combined with invasiveness of traditional surgical ventricular assist device placement, have stimulated the development of percutaneous mechanical circulatory support (MCS). Impella® (Abiomed, Danvers, Massachusetts, USA) and TandemHeart® (CardiacAssist, Inc., Pittsburgh, Pennsylvania, USA) are prototypical devices in this category.
Advantages of percutaneous MCS include deployment without the traditional surgical approach, thus sparing operating room resources and time, relative simplicity of insertion, and ability to rapidly institute mechanical assistance, thereby expediting resuscitation.
The Impella® consists of a micro-axial pump mounted on a 9 French catheter. The device is situated in the left ventricle, having crossed the aortic valve. Blood is drawn into the pump through an inlet, and then ejected beyond the aortic valve through an outlet, into the ascending aorta. An external console controls and monitors speed and pressure measurements, ensuring appropriate pump function.
Multiple configurations of the Impella® are available. Currently, the 2.5 (2.5 lpm, 12 French pump) and CP (14 French pump) are percutaneously insertable. The 5.0 (5.0 lpm, 21 French pump) and LD (left direct – 5 lpm, 21 French pump) require a surgical approach. The 5.0 is inserted via a graft anastomosed to the femoral or axillary artery, while the LD is placed through a graft sewn on the ascending aorta and directly inserted in LV.
The Impella® 2.5 is unlikely to provide sufficient decompression and cardiac output for the severest cases of cardiogenic shock and postcardiotomy shock (PCS). The Impella-EUROSHOCK Registry found a greater than 64 % 30-day mortality in post-myocardial infarct cardiogenic shock supported with Impella® 2.5 [11]. The Impella® 5.0 and LD, however, have shown some potential utility in the PCS cohort [12].
Contraindications to insertion of this device include presence of a mechanical prosthetic aortic valve, significant aortic stenosis, aortic regurgitation, atherosclerotic aortic, and left ventricular (LV) thrombus [13]. Complications particular to this pump to consider include arrhythmias, aortic insufficiency, LV perforation, lower extremity ischemia, and pump migration. Hemolysis and intraventricular thrombosis have also been reported with Impella® usage [14–16].
The TandemHeart® system consists of a paracorporeal centrifugal pump, requiring only 10 ml priming volume, a transseptal left atrial cannula, and a femoral arterial cannula. Thus, left atrial to femoral artery bypass is accomplished, entirely percutaneously. This system is able to deliver up to 4–5 lpm cardiac output while decompressing the left heart [17, 18]. Utility of this system has been reported in a variety of clinical scenarios, achieving satisfactory hemodynamic support [19]. The interatrial septal puncture does not require closure – although it is surgically repaired if the patient is transitioned to long-term VAD. While device insertion is performed in the cardiac catheterization lab, the TandemHeart® is removable at the bedside.
Significant aortic insufficiency is a contraindication to specifically employing the TandemHeart®. Device-specific complications include lower extremity ischemia, cannulae migration, persistent atrial septal defect, and left atrial perforation [13].
19.5 Short-Term Ventricular Assist Devices (VADs)
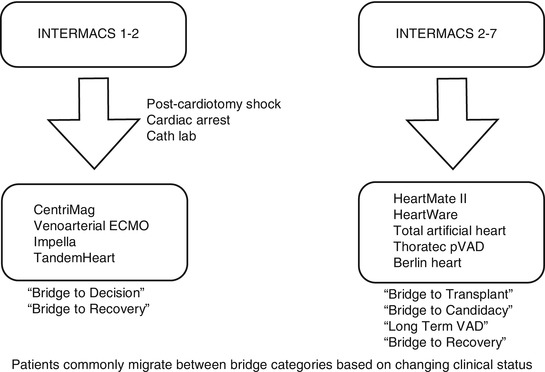
Decisions regarding MCS implantation can be challenging for INTERMACS profile 1 or 2. Time limitations based on critical clinical conditions may not permit detailed evaluation to determine long-term MCS or transplantation suitability. Short-term VADs are an appropriate “bridge to decision” strategy, whereby a trial of MCS may provide an opportunity to ascertain end-organ dysfunction reversibility, especially if neurologic status is unclear. This is particularly applicable for postcardiotomy shock (PCS), cardiac arrest scenarios, or cardiac catheterization lab support. Historically dismal PCS outcomes have improved substantially with VAD support [20].
The ideal short-term VAD should be relatively inexpensive and capable of rapid, easy deployment. Simplicity of management is also desirable. Percutaneous devices fulfill these requirements. Currently, these devices have limitations with duration of support. Patient immobility is another consideration. Most importantly, however, the option to readily provide biventricular support is desirable. Right ventricular percutaneous support systems are still under development and evolution. Additionally, percutaneous VAD support systems are suboptimal choices for more intermediate durations of support.
At the Mazankowski Alberta Heart Institute, we employ the CentriMag® (Thoratec®, Pleasanton, California, USA) paracorporeal support system. This device provides the option to provide isolated left or right ventricular assistance or biventricular support [21–24]. Takayama et al. have described a percutaneous strategy for deploying the CentriMag® as an RVAD [25].
When cannulating the patient centrally, the cannulae are tunneled and exit through the anterior abdominal wall. The actual pump rests within a bearingless motor, connected to the drive console. We have also successfully employed a temporary in-line oxygenator when hypoxemia is not manageable with mechanical ventilation alone. As pulmonary edema resolves, and hypoxemia improves, the oxygenator can be readily removed from the circuit.
The CentriMag® system is magnetically levitated, bearingless, and capable of generating up to 10 l/min of flow at a maximum of 5,500 rpm. Without bearings, regions of blood stasis and friction, thermal damage, hemolysis, and thrombus formation are reduced [22].
Temporary VAD implantation is recommended (class IIa) for patients in cardiogenic shock with end-organ compromise or unclear transplant eligibility status, who have a reasonable expectation to improve with restoration of good hemodynamics [26].
19.6 Long-Term VADs
For long term destination may be considered as a bridge to transplant, bridge to candidacy, bridge to recovery, or for long-term/destination therapy. Increasingly, patients may migrate between these broad categories, based on medical status, personal preference, and technological advances. Early referral for VAD is always preferable. Suitable candidate selection requires recognition of patients too ill to benefit, balanced against situations where patients are not ill enough.
Currently, implantable continuous-flow pumps have supplanted volume-displacement (pulsatile) devices in most adult assist device programs. Continuous-flow devices are quieter, have greater ease of implantation, and are of significantly smaller size. Continuous-flow ventricular assist devices (CF-VADs) still require anticoagulation, typically with warfarin and ASA. Patients bridged to heart transplantation with CF-VADs have similar posttransplant survival at 1 and 3 years (87 and 82 %, respectively) compared to non-LVAD-bridged recipients not on inotropic support (88 and 82 %) [27].
With respect to long-term implantation, when compared to pulsatile devices, continuous-flow LVADs, CF-VADs, have been shown to have better outcomes with respect to stroke and 2-year survival [28]. Besides better device durability, CF-VADs also have 50 % fewer device-related infections [28]. CF-VADs improve both functional capacity and quality of life based on heart failure metrics [29]. Battery technology continues to improve, permitting increasing freedom for these patients. While pulsatile devices provide greater left ventricular volume unloading, there is no difference in hemodynamic support or exercise capacity based on VAD design alone [30].
The HeartMate II® (Thoratec®, Pleasanton, California, USA) axial-flow rotary blood pump is currently the most popular durable continuous-flow implantable left ventricular assist device (LVAD) and has been effective for bridge to transplant (BTT) and permanent or destination therapy (DT) [2, 31, 32]. The device currently has US Food and Drug Administration (FDA) approval for bridge to transplantation, as well as destination therapy.
An inflow cannula is inserted into the left ventricular apex, with an outflow graft anastomosed to the ascending aorta. The device is implantable and rests subdiaphragmatically either intra-abdominally or in the pre-peritoneal space of the left upper quadrant. Blood leaves the left ventricle and enters the pump through an inflow conduit. An electric motor drives a permanent magnet, the rotor. As the rotor spins, blades propel blood through the outflow graft back into the ascending aorta. The HeartMate II® is an axial-flow pump; that is, blood flow enters and exits parallel to the pump axis.
The rotor spins on bearings, capable of generating as much as 10 l per min blood flow, functioning in parallel with the patient’s circulation. Clinicians set a fixed speed for the pump, generally between 8,000 and 10,000 revolutions per minute (RPM), and actual flow depends upon various factors, including patient afterload, pump speed, and power provided to the motor. A system controller, worn around the patient’s waist, is connected to the pump by a transcutaneous driveline and regulates device function. Portable batteries allow patients to mobilize untethered [33].
Long-term implantation with this rotary device has been shown to have fewer complication, improved survival, better quality of life, and improved functional capacity compared to a pulsatile VAD [29]. The European experience with this device has demonstrated similar excellent outcomes and durability for long-term support [34]. Destination therapy patients have improving 1-year survival, around 74 % [32].
Our center also employs the HeartWare® ventricular assist system (HVAD®) (Framingham, Massachusetts, USA). The HeartWare® device currently has FDA approval for bridge to transplant indications. This pump sits within the pericardial space. Ease of implantation is enhanced not only by eliminating the need to dissect below the diaphragm but also by simplicity of actual implant technique. Centrifugal in design, the rotor (often referred to as the impeller), is suspended by magnets and hydrodynamic thrust bearings. There are no points of mechanical contact within the pump. The pattern of blood flow is similar to that described above for the HeartMate II®: blood enters the device through an inflow cannula integrated within the pump. The suspended impeller drives blood forward, exiting via the outflow cannula and through a graft anastomosed onto the ascending aorta. A percutaneous driveline is tunneled from the device to a controller worn around the patient’s waist. Portable batteries permit patient mobility and freedom from tethering.
Blood flow is determined by impeller speed (RPMs), current (power), and blood viscosity. Typically, the device is set to operate between 2,400 and 3,300 RPM. Additionally, clinicians enter the patient’s hematocrit, and blood viscosity is calculated from this value. As with the HeartMate II®, blood flows are estimated.
Typically employed as a left-sided VAD, the HeartWare® HVAD® has been implanted as an isolated RVAD [35] as well as a permanent implanted biventricular support system (BiVAD) [36, 37]. Potential use as a BiVAD is advantageous; however, quality of life is significantly different with two devices, two controllers, and two sets of batteries to manage, compared to a simple LVAD.
Potential disadvantages with the HeartWare® HVAD® device include higher recommendations for anticoagulation, along with thrombosis concerns [38–40]. Lower rates of thrombosis have been described more recently, possibly attributable to an improved LV coring tool and a sintered inflow cannula [39, 40]. HeartWare® recommends targeting a PT INR of 2–3 for the HVAD® [41], compared to a PT INR of 1.5–2.5 for the HeartMate II® [42]. Additionally, although there are considerable differences between centers and local protocols, recommendations for antiplatelet therapy are generally greater with the HVAD® than the HeartMate II® [43].
There are notable physiological differences between axial and centrifugal pumps [44]. While both types of rotary pumps are afterload sensitive, centrifugal pumps have greater afterload sensitivity compared to axial-flow devices. Centrifugal pumps also have greater flow pulsatility and higher estimated flow accuracy. During lower flow conditions, centrifugal flow devices typically demonstrate lower inlet suction.
Beyond survival, end-organ optimization and functional recovery are the goals of MCS. Patients with established renal failure who are unlikely to recover function despite improved cardiac output are not recommended as candidates for long-term devices [26]. CF-VADs enhanced functional capacity and quality of life [28] compared to pulsatile devices [45]. Health-related quality of life and functional capacity assessment, rather than survival, are increasingly pertinent. Improved understanding of these factors may be useful in determining patient-device suitability and lifestyle modifications, and perhaps refining implantation indications [46].
Neurologic events are still reported in nearly 10 % of HeartMate II® recipients [47]. Ischemic strokes occur in 8 % of recipients, while 11 % may suffer a hemorrhagic stroke [28]. Subsequently, less aggressive anticoagulation regimens have been advocated.
Development of aortic insufficiency (AI) has been identified as an issue with continuous-flow long-term VADs. Nearly half of patients demonstrate moderate or greater AI by 18 months after CF-VAD implantation [48]. For this reason, if possible, maintaining aortic valve opening by permitting some left ventricular loading may be desirable.
Use of CF-VADs has uncovered a specific hemodynamic and hematologic constellation that can result in hemorrhage in 25 % of patients [49]. Significant epistaxis is an etiology in one-fifth of cases [49]. Gastrointestinal bleeding is common – over 20 % – following CF-VAD implantation [50, 51]. Angiodysplasia and arteriovenous malformations (AVM), coupled with anticoagulation, acquired von Willebrand factor (vWF) deficiency, fibrinolysis, and reduced platelet numbers with impaired function, all contribute to bleeding [52–55].
Two mechanisms are proposed to explain AVM. Firstly, it is postulated that increased intraluminal pressure and vascular smooth muscle contraction results in increased smooth muscle tone and vessel dilation, with ensuing AVM formation. The second mechanism supposes that reduced pulse pressure results in hypoperfusion, vascular dilation, and ultimately angiodysplasia [56].
Analogous to Heyde’s syndrome, the shear stress produced during CF-VAD therapy results in reduction of high molecular weight (HMW) vWF multimers [52, 57]. The pump itself may directly contribute to HMW vWF deformation and proteolysis. vWF binding to collagen or platelet gp1b receptor binding is thus impaired. Multiple mechanisms of acquired vWF deficiency have been elucidated from in vitro studies [58].
Driveline infections remain problematic: the Mayo Clinic reported a 12 % driveline infection rate, with prolonged duration of support increasing the risk [59]. Based on INTERMACS data, nearly one-fifth of patients experience a driveline infection within a year of LVAD implantation. Interestingly, younger age represents the only identifiable risk factor. Most concerning is that driveline infections may be adversely associated with survival [60].
Patient selection is becoming increasingly refined with greater clinical experience. Various scoring systems have emerged to assist clinical decision-making. Recently, the HeartMate® II Risk Score (HMRS) has been proposed as a mortality risk stratification tool for CF-VADs [61]. Patient age, serum albumin, serum creatinine, INR, and center volume are predictive factors. The Destination Therapy Risk Score (DTRS) [62] was developed during the pulsatile device era and is more complex to calculate. DTRS utility in the CF-VAD era is likely limited. MELD scoring has also been successful at predicting mortality for CF-VADs [63–65].
VAD usage as a bridge to transplantation is currently a class I recommendation for patients who have failed maximal therapy and have a high risk for mortality prior to allograft availability [26]. Early referral for VAD implantation is currently a class IIa recommended approach, as outcomes are superior prior to the patient’s developing hypotension, hyponatremia, renal dysfunction, and the need for recurrent hospitalizations [26]. Increasingly outpatients are being evaluated for appropriateness of mechanical circulatory support (MCS) [66].
Patients deemed ineligible for cardiac transplantation due to pulmonary hypertension may benefit from hemodynamic unloading of the left ventricle. Coupled with aggressive medical therapy, long-term VAD often successfully bridges this patient group to transplant candidacy by reducing pulmonary artery pressures, transpulmonary gradient, and pulmonary vascular resistance [67]. The current recommendation for bridge to candidacy with long-term VAD for pulmonary hypertension related to HF is class IIa [26].
Permanent or destination therapy (DT) with a pulsatile LVAD was first demonstrated to be a feasible alternative to medical therapy for end-stage heart failure in the REMATCH trial [45]. Subsequently, it was advocated that LVAD therapy for DT yielded better results if implantation was performed prior to development of major complications [62]. Currently, durable LVAD placement is advised (class I) for transplant-ineligible advanced heart failure patients with a high 1-year mortality risk, without irreversible end-organ dysfunction [26]. Elective implantation is also advocated over urgent VAD (class IIa) [26].
Prior to acceptance for long-term VAD implantation, a multidisciplinary team assessment (surgical, medical, nutritional, and psychosocial) is highly recommended (class I) [26]. In our experience, participation of cardiology, cardiac surgery, critical care, and a specialized VAD team has greatly improved assessment, decision-making, communication, and management.
19.7 Right Ventricular Failure (RVF)
Medical management of RVF is beyond the scope of this chapter. However, RV function is a critical consideration during MCS application. RVF is associated with higher earlier morbidity and mortality [2, 68]. Unlike venoarterial ECMO, LVADs do not directly unload the right heart. Nevertheless, following LVAD implantation, objective improvements in RV function are detectable [69]. Conversely, CF-VADs may exacerbate RVF by causing interventricular septal shift, RV distortion, and worsening tricuspid regurgitation, combined with increasing RV preload. RVF that requires temporary RVAD support may occur in up to 9 % of LVAD recipients, with an associated significantly increased mortality [70].
Predicting RV failure, therefore, has important clinical implications. The right ventricular failure risk score relies upon four variables (vasopressor usage, creatinine, bilirubin, and aspartate aminotransferase) as predictors of post-LVAD implantation RV failure [71]. A higher RVFRS was also associated with greater mortality [71]. Other investigators have reported preoperative tricuspid regurgitation as predictive of RV failure [68]. Raina and colleagues describe an echocardiographic scoring system consisting of RV fractional area change, estimated right atrial pressure, and left atrial volume index [72]. Central venous pressure (CVP) to pulmonary capillary wedge pressure ratio greater than 0.63, elevated blood urea nitrogen, and preoperative mechanical ventilation have been shown to be independent predictors of RVF following CF-VAD insertion [73]. These investigators also found elevated CVP (>15 cm H2O) and right ventricular stroke work index <300 mmHg × ml × m−2 to be predictive.
19.8 Total Artificial Heart (TAH)
TAH technology offers biventricular support in the form of an orthotopically placed device, which necessitates complete biventricular and heart valve excision [74]. The SynCardia® temporary TAH (TAH-t) (SynCardia Systems, Inc., Tucson, Arizona, USA) is a pneumatically driven, volume-displacement device, typically delivering between 7 and 9 l/min of cardiac output [75]. It has been used as a bridge to transplant with 61–87 % success rate [74–77].
There is limited clinical experience with the AbioCor® (Abiomed®, Massachusetts, USA), an entirely self-contained TAH. The internal battery is rechargeable using transcutaneous energy transmission (TET). In the USA, the device has FDA approval as Humanitarian Use Device. A next generation device is under development.
TAH is an attractive option for MCS in the setting of cardiac allograft failure, as donor graft ventricles are excised, eliminating the need for immunosuppression, and thus the associated risks of wound healing and sepsis [78, 79]. Other unique clinical scenarios where TAH support is potentially advantageous include post-infarct ventricular septal defect [75, 80] and massive myocardial infarction/necrosis. Furthermore, in patients requiring biventricular support as bridge to cardiac transplantation, TAH had a significantly lower stroke rate and a trend toward improved survival when supported for greater than 90 days [81].
Currently, limitations of TAH technology include device size and complication rates.
19.9 Transplantation
The gold standard treatment for end-stage heart failure remains cardiac transplantation. Graft durability, however, remains limited by accelerated cardiac allograft vasculopathy (CAV). Median recipient survival remains 10 years [27]. Additionally, the organ supply-demand discrepancy continues to grow. Since 1998, less than 4,000 heart transplants have been reported per annum to the International Society for Heart & Lung Transplantation (ISHLT) Registry, and these data probably represent two-thirds of heart transplants performed globally [27]. Interestingly, 36 % of heart transplant recipients are currently bridged with MCS [27].
Besides limited donor organ availability, post-transplantation challenges exist. Prolonged immunosuppressive therapy is required. Donor right heart failure following transplantation remains a significant concern due to its frequency and association with poor outcomes. In the first 3 years following cardiac transplant, graft failure and infection represent the most common causes of death. Beyond 3 years, malignancy and cardiac allograft vasculopathy contribute to mortality [27].
Refractory, end-stage heart failure patients are recommended for cardiac transplant referral [81]. Patients in cardiogenic shock with documented inotropic dependence, peak VO2 (oxygen consumption) less than 10 ml/kg/min, severe symptomatic ischemic heart disease not amenable to revascularization, and refractory ventricular arrhythmias are all suitable cardiac allograft candidates [82]. Additionally, patients on MCS with device-related complications should be advanced for transplantation. Canadian Cardiovascular Society guidelines recommend MCS for cardiac transplant candidates who clinically deteriorate or are unlikely to survive until a suitable donor organ becomes available [83].
Decisions to proceed with listing for transplantation may be aided by an estimation of mortality risk. Adjudging heart transplant candidacy in ambulatory patients includes measuring VO2, calculating the Heart Failure Survival Score, and employing the Seattle Heart Failure Model [84].
Heart transplantation is contraindicated for patients with active malignancy or infection, as both conditions may be exacerbated by immunosuppression. Pulmonary hypertension heralds poor outcomes following transplantation. Pulmonary vascular resistance (PVR) >5 Wood units or a trans pulmonary gradient (TPG) ≥15 mmHG are contraindication to cardiac transplantation [85]. European guidelines suggest that a PVR >4–5 Wood units and a transpulmonary gradient >15 mmHg are contraindications [86].
Recently, the Columbia group has published the CARRS prognostic scoring system to predict survival in high-risk transplant candidates. CARRS incorporates cerebral vascular accident, serum albumin, retransplantation, renal dysfunction, and >2 prior sternotomies as risk factors. They found that a high score was predictive of poorer survival [87].
Currently, over one-third of patients are bridged by MCS to heart transplant [27]. Of the current MCS options, LVAD support as a bridge to transplant provides the best outcomes [88]. It is recognized that cardiac transplantation for INTERMACS 1 and 2 patients is associated with poorer outcomes than bridging with MCS. Attisani and colleagues reported 42.3 % early mortality for INTERMACS profile 1 and 2 patients undergoing urgent cardiac transplantation versus 4.3 % for emergent MCS insertion [89]. The Spanish National Heart Transplant Registry database was recently examined and the investigators demonstrated that INTERMACS profile correlated with outcomes following emergency heart transplantation: postoperative mortality was 43 % in profile 1 patients and 26.8 % in profile two recipients [90].
Enthusiasm for a possible future with limitless donor organs was fostered by the clinical case of baboon-to-human cardiac xenotransplantation in 1985 – “Baby Fae” [91]. The eagerness for cardiac xenotransplantation, however, abated following recognition of the potential for xenozoonoses, with an unknown actual transmission risk [92]. Furthermore, immunologic barriers have not been overcome, and primary graft dysfunction following cardiac xenograft remains a challenge [93].
Globally, insufficient access to donor organs exists to meet the demand for heart transplantation. Additionally, in the MCS era, some patients decline the opportunity for a heart transplant once they have become accustomed to improved quality of life compared to their previous existence. The evolution of VAD technology in the continuous-flow era has led to the suggestion that long-term mechanical assist device support outcomes are rapidly becoming on par with heart transplantation [94].
As patient selection and technology are refined, risks and complications will be further reduced, and MCS may become preferred to heart transplantation in certain scenarios. Since cardiac allografts have a finite lifespan – median recipient survival of 10 years – it may be more desirable to perform VAD implantation in young patients, with device replacement as required, reserving the limited donor cardiac grafts until later in the course of the disease process or until needed to overcome device-related complications.
19.10 Algorithm
The key to salvageability is early, expeditious intervention. The Minnesota program has described their bridge to decision approach for refractory cardiogenic shock patient with multiple organ dysfunction [18]. They employ CentriMag® BiVAD support and reevaluate future decisions based on end-organ recovery, neurologic status, and cardiac recovery.
Figure 19.1 outlines a proposed algorithm for device selection based on INTERMACS profile level and clinical scenario. Since clinical status determines patient categorization, migration between categories is common. Accordingly, a “bridge to bridge” strategy may also be employed. For example, VA ECMO may be used as bridge to a short-term device, which could in turn serve as a bridge to decision.