Reference
Year
No. of patients
Bridge duration (days)
Type of ECMO
Successful bridge (%)
1-year survival (%)
Fischer et al. [8]
2006
12
15 ± 8 (4–32)
AV
83
80
Cypel et al. [9]
2010
10
5 (1–25)
VA (3), VV (2), AV (4), PA-LA (4)
100
70
Ricci et al. [10]
2010
12
13.5 ± 14.2 (4–48)
AV (6), Decap (6)
25
NA
Hammainen et al. [11]
2011
16
16.8 ± 19.2 (1–59)
VV, VA
81
92
Bermudez et al. [12]
2011
17
3.2 (1–49)
VV (8), VA (9)
NA
74
Fuehner et al. [13]
2012
26
9 (1–45)
VV (14), VA (12)
77
80 (6 months)
Lang et al. [14]
2012
34
4.5 (1–63)
VV (18), VA (14), AV (1), comb (4)
76
60
Javidar et al. [15]
2012
18
11.5 (6–18)
VV (13), VA (5)
72
100 (3 months)
Shafii et al. [16]
2012
19
6.5 (1–16)
VV (11), VA (8)
74
75
Toyoda et al. [17]
2012
31
7.1 ± 10.1 (0.1–46)
VV (15), VA (9), 7 NA
77
74
Hoopes et al. [18]
2013
31
11 (2–53)
VV (13), VA (12) PA-LA (3), comb (4)
NA
92
Crotti et al. [19]
2013
25
24 ± 31 (1–157)
VV (19), VA (2), AV (4)
68
76
25.2 Indications, Timing, and Patient Selection
The main indications for the ECMO bridging to lung transplant include all the irreversible end-stage respiratory diseases, which have a rapid worsening of the respiratory function, as well as severe pulmonary hypertension with right-sided ventricular failure.
So far, there is no evidence regarding the correct timing of the artificial respiratory support. Some centers start the ECMO bridging when the clinical condition deteriorates to the point that the patient’s life expectancy could be considered less than 24–48 h without intubation and/or extracorporeal support.
Careful patient selection is needed to maximize the results of ECMO bridging and to avoid a waste of viable donor lungs. In our center we have applied ECMO bridging in LTx candidates in whom respiratory failure is the sole relevant organ failure (aside from right-sided heart failure) and in whom no exclusion criterion for LTx or ECMO is present.
The most common behavior is to use this therapeutic option for patients: already candidates for lung transplant, young, free from other organ failures, and with a good expectancy of physical recovery after transplant. This is because factors, such as age, organ dysfunction, some infections, and physical status, are risks for postoperative death, even for patients on the standard list [1]. It is worth noting that the postoperative survival of patients is affected by the recipient’s age. In fact recipients older than 55 have a significantly higher risk of death at 1 year after transplant [1].
The coexistence of other organ dysfunctions alongside the end-stage respiratory failure and/or pulmonary hypertension decreases the 1-year survival after LTx. Particularly the need of hemofiltration and the use of inotropic drugs in the perioperative period are risk factors for the 1-year mortality [1].
Septic shock is a contraindication for lung transplants, whereas the presence of leukocytosis and fever in the immediate preoperative period slightly increases the risk of death for postoperative sepsis [20]. In CF patients, the pre-transplant pulmonary colonization with Burkholderia cepacia genomovar III increases postoperative mortality, whereas the colonization with other B. cepacia strains, or multi- or pan-resistant Pseudomonas aeruginosa, or methicillin-resistant Staphylococcus aureus, or Aspergillus fumigatus, does not affect postoperative survival [20].
Investigations of muscle function in lung transplant recipients reveal decreased muscle mass and strength with a persistent limitation in exercise capacity at 1 year after LTx. Pre-existing peripheral muscle dysfunction in chronic lung disease is one of the determinants of the postoperative impairment in physical status, suggesting a need for physical therapy to optimize muscle strength and functional capacity during the pre-transplant period [21].
25.3 ECMO Configuration
Deciding the type of extracorporeal support must take into account the characteristics of the respiratory failure, the presence of pulmonary hypertension, and the concomitant right-sided heart failure. The ECMO approach and the specific device chosen will fit with the clinical patient’s condition (Fig. 25.1).
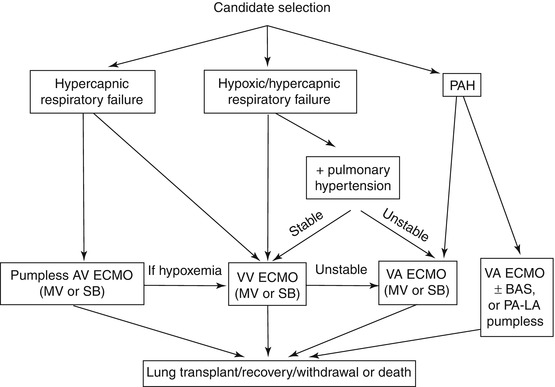

Fig. 25.1
Algorithm for selection of ECMO support configuration
Most end-stage respiratory diseases, requiring lung transplant as a unique therapeutic option, lead to a mainly hypercapnic respiratory failure. When the noninvasive trial fails, the extracorporeal support becomes a valid option to bridge these patients to LTx. In the hypercapnic patients, the two configurations most often used are the pumpless arteriovenous (AV) and the venovenous VV ECMO.
The pumpless arteriovenous (AV) approach has been recently utilized successfully. The first report is by Fisher et al. who described the use of the “novel pumpless device” in 12 patients between 2003 and 2005 [8]. They reported a very successful bridge to LTx (10 of 12 patients underwent a transplant) and an 80 % 1-year survival. Other groups experienced the low blood flow – CO2 removal devices reporting different successful rate. Ricci et al. described 12 patients treated with pumpless AV or decap navigation system Decap [10]. They were able to reverse the respiratory acidosis, but 8 of 12 patients died prior to transplant. Cypel et al. recently published four patients successfully bridged to LTx with the AV mode and another four patients that required a conversion to a VV or venoarterial VA ECMO during the bridging period [9].
In the AV ECMO setting, the blood is driven through the circuit by the difference between the femoral arterial pressure and the venous reinfusion pressure. This requires an adequate patient’s mean arterial pressure. Moreover, in AV mode the ECMO blood flow cannot be actively changed (maximum value of 1–1.5 L/min), limiting the extracorporeal oxygen supply. If the patient’s oxygenation drops during the bridge period, a switch to a VV configuration becomes necessary, with possible bleeding problems at the arterial cannula removal site. In AV mode, heart performance must be good enough to increase the patient’s cardiac output as requested by the high-flow “fistula”, as the AV ECMO could be considered. Nevertheless, AV mode offers an optimum and effective CO2 removal that can be titrated by changing the sweep gas flow from a minimum level up to 12 L/min.
Some end-stage respiratory failure could be both hypercapnic and hypoxic. The VV configuration permits controlling respiratory acidosis through an adequate CO2 removal and provides oxygen supply by varying the ECMO blood flow up to 4–5 L/min. If recirculation is minimized, even in the presence of severe hypoxic disease, the VV mode could be feasible in managing the patients until a suitable organ becomes available. Most of the transplant centers have recently increased the use of this approach for all the patients without right-sided heart failure. Up until 2007, only single-center case reports had been published [22]. In the last 4–5 years, many centers around the world have reported an increased experience [8–19]. As shown in Table 25.1, in which the case series of more than ten patients are listed, the VV approach is the more frequently used in each center. The VV configuration could also support blood gasses during the intraoperative management of the patient, and, whenever possible, it should be preferred to a central VA bypass. This offers some advantages, such as less need of intraoperative anticoagulation, simpler technical management, and better evaluation of graft performance after LTx.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


