Common indications
Cardiogenic shock
Refractory cardiac arrest
Impossible weaning from cardiopulmonary bypass during cardiac surgery
Myocarditis
Intoxication or sepsis with severe myocardial depression
“Primary graft failure” after heart or lung transplant
Purposes of extracorporeal support
“Bridge to recovery” – assistance until recovery and weaning from support
“Bridge to bridge” – until the implantation of medium- or long-term mechanical support (VADs)
“Bridge to transplant” – until heart transplant
“Bridge to decision” – to evaluate the possible cardiac recovery and the most appropriate therapy
At the same time, the intensivist has become more familiar with echocardiography and ultrasound techniques even outside the traditional field of cardiac surgery, making it possible an “ultrasound-guided approach” to the indication, correct positioning, and patient monitoring during extracorporeal assistance. The targets of ultrasound monitoring during extracorporeal circulation will be different depending on which approach (VV versus VA) has been used. During circulatory assistance, after the patient has been selected and the cannulation sites have been evaluated, the attention will be focused on the correct functioning of the mechanical support (position of the cannulae, thrombus formation, etc.) and on the cardiac function, monitoring its recovery and deciding when the patient is ready to be disconnected from support. During respiratory support, after assessing the proper positioning of the cannulae, it is important to evaluate the cardiocirculatory status of the patient before and during the assistance.
The echocardiographic approach, transthoracic (TTE) versus transesophageal (TEE), will depend on the patient’s characteristics (acoustic windows and echogenicity) and on the region of the heart and vessels of interest. Ultrasound monitoring during ECMO is not only important for cardiac evaluation but also for choosing the best sites for cannulation (especially during percutaneous procedures) and for monitoring the peripheral perfusion of the cannulated limbs.
Despite its important role in the management of critically ill patients, there are few published data outlining the use and experience of echocardiography and ultrasound in critically ill adults requiring extracorporeal support. Because ECMO is based on the principles of oxygenation and hemodynamic support via blood flow within large-bore cannulas placed in or near the heart in patients with cardiorespiratory failure, echocardiography would be expected to have a fundamental role throughout the care of patients supported on ECMO. In this chapter, we outline the targets and the modalities of ultrasound evaluation in patients requiring extracorporeal assistance.
31.2 Venoarterial ECMO
As pointed out in the previous paragraph, the primary aim of VA ECMO is to support the circulatory function in patients with cardiac failure (acute or chronic, acutely decompensated) of different etiologies. Besides this traditional indication, in the last decade, venoarterial ECMO has emerged as a circulatory and respiratory support for refractory cardiac arrest, allowing diagnostic evaluation and treatment in selected patients that would have a mortality rate close to 100 %.
Compared to other mechanical cardiac assistance devices, ECMO has the advantage of reduced costs, and the possibility of being easily and rapidly instituted outside the operating room (critical care units, cardiac catheterization suites, or emergency departments) even during cardiopulmonary resuscitation maneuvers. It also has limitations: it is a short-term support, with important infectious, thrombotic, and hemorrhagic complications, and causes an increase in the left ventricle afterload.
In the next paragraphs, we are going to analyze in details how a comprehensive ultrasound evaluation should be performed during the different phases of ECMO support. For cannulation procedures, we refer to the peripheral cannulation, which is employed in the majority of patients. Central cannulation (right atrium to ascending aorta) represents a specific condition, reserved almost exclusively to the intraoperative cardiac surgery period (impossible weaning from extracorporeal circulation). In these cases, the ultrasound evaluation is represented by the intraoperative transesophageal examination performed to assist the weaning from cardiopulmonary bypass.
31.3 Indications to VA ECMO Support
31.3.1 Cardiogenic Shock
Patients with cardiogenic shock should be carefully evaluated: A comprehensive clinical history and examination together with a complete echocardiographic exam are necessary to determine their functional status as well as the presence of clinical conditions which can contraindicate the positioning of a circulatory assistance (Table 31.2). Echocardiography helps to exclude new reversible pathologies, which may account for a patient’s hemodynamic instability (such as cardiac tamponade, undiagnosed cardiac valve pathology, and LV dysfunction), avoiding the need for ECMO support. During the exam, it is important to study both the systolic and the diastolic function of the left ventricle, the function and dimensions of right ventricle, the valves (searching for functional or anatomic anomalies), and the pericardium, focusing the attention to the presence of pericardial effusion. It is of utmost importance to accurately evaluate the aortic valve and the aorta (ascending and descending): The presence of severe aortic valve regurgitation may have a detrimental impact on left ventricle unloading once a VA ECMO (in which LV afterload is increased) is positioned, and the detection of an aortic dissection is an absolute contraindication for VA ECMO positioning. The positioning of a venous cannula in the right atrium also dictates that right-heart anatomy be evaluated for any structural abnormality that may adversely affect the function and positioning of the cannula. Notable findings include a prominent patent foramen ovale, atrial septal defect, interatrial septal aneurysm, prominent Chiari network, presence of a pacemaker or implantable cardioverter-defibrillator leads, and tricuspid valve pathology (such as tricuspid stenosis or a tricuspid valve replacement).
Table 31.2
Contraindications to ECMO support
General |
Severe, not reversible cardiac insufficiency with no indication to transplant or VAD as destination therapy |
Severe, not reversible neurological injury |
Terminal malignancy |
Intracranial bleeding |
Agea |
Venoarterial ECMO |
Aortic dissection |
Severe aortic valve regurgitation |
Not witnessed cardiac arrest |
Prolonged CPRb |
Venovenous ECMO |
Cardiac arrest |
Cardiogenic shock |
Severe pulmonary hypertension |
31.3.2 Refractory Cardiac Arrest (Fig. 31.1)
Fig. 31.1
ECMO cannulae positioning during CPR
The criteria to establish the indication for ECMO assistance in patients with cardiac arrest are primarily based on the clinical history and on the timing of CPR start (i.e., the duration of no-flow or non-assisted circulatory arrest) (Table 31.2). In this category of patients, the echocardiographic evaluation is limited to the anatomic aspects of cardiac chambers and valves, the study of cardiac function being impossible. In PEA (pulseless electrical activity) patients, it is important to distinguish a true PEA (the heart has no contractions, “electromechanical dissociation”) from a pseudo-PEA (the heart has contractions which are not sufficient to generate a significant pulse because of a severe dysfunction or extrinsic compression). Especially in these patients, it is important to look for clear and gross anomalies which can explain the cause of the arrest (the 4 Hs and 4 Ts of the ALS algorithm) focusing the attention on the dimension of the cardiac chambers – especially of the right ventricle to exclude pulmonary embolism – and on the presence of significant pericardial effusion. It is also possible to evaluate the efficacy of the thoracic compressions estimating the transaortic or transpulmonary flow. In these patients, it is also important to exclude clinical conditions – aortic dissection in primis – which contraindicate cannulation.
31.4 Cannulation
Ultrasounds also have a key role during ECMO cannulation, as they assist in the correct placement of ECMO cannulas. The evaluation of arterial and venous vessels is important for choosing the best site for cannulation. With ultrasounds, it is possible to determine the position of the veins and arteries, the potential anatomical variants, as well as the presence of significant pathologies of the venous (thrombosis) or arterial system (occlusion, significant stenosis, aneurysms, atheromas). If a significant pathology is found in the examined vessels, alternative sites can be evaluated to determine the most appropriate for cannulation. This aspect has to be investigated as quickly as possible, especially in cardiac arrest patients.
The evaluation of vessels size, especially of the arteries, is important for choosing the dimension of the cannulae; the size of the arterial cannula is the major determinant of the maximal output achievable with ECMO support, as larger cannulae permit a greater output. In our experience with adult patients, it is possible to achieve a total circulatory assistance with cannulae ranging from 15 to 19 French depending on the size of the patient. It is easy to obtain the approximate dimensions of the cannula in millimeters, dividing the size in French by 3. From this calculation, it is clear that we can safely cannulate arteries with a diameter greater than 5–5.5 mm. The ultrasound examination of the artery we intend to cannulate can tell if its diameter is sufficient to accommodate the smallest cannula able to provide an adequate blood flow to the patient. The presence of a vessel that is close in size to the cannula dimension warrants the positioning of a peripheral reperfusion cannula to prevent ischemia of the cannulated limb.
When commencing cannulation, guidewires are initially inserted percutaneously and positioned within the heart or great vessels, before the advancement of cannulas over these wires. Because of the strong echocardiographic artifacts that can be generated from these wires and cannulas, close attention must be paid to their placement.
In peripheral VA ECMO, the venous cannula is optimally located in the mid right atrium to provide unobstructed flow of central venous blood into the circuit. Transesophageal echocardiography (TEE) is useful to guide positioning. The return cannula is usually placed in the contralateral femoral artery and the tip located in the iliac artery or abdominal aorta. This region cannot be visualized with TEE. However, imaging for placement of this cannula is usually not required. TEE can confirm that the guidewire used in percutaneous arterial cannulation is in the lumen of the aorta before dilatation, reducing the risk for extra-arterial cannula placement.
31.5 Monitoring ECMO Support
During ECMO support, the echographic evaluation – performed at least on a daily basis – is of utmost importance for the study of cardiac function as well as for monitoring the assistance and managing the complications. All these aspects are investigated during all the period of assistance that can be divided into three stages: the initial phase after connection, the intermediate of maintaining support, and the final of weaning and de-connection.
31.5.1 Initial Stage (After Connection)
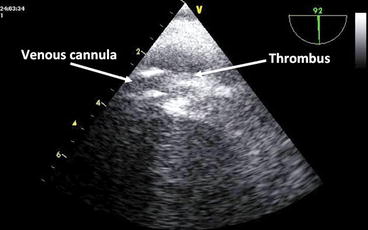
After ECMO connection and the beginning of assistance, it is important to verify if the venous drainage is sufficient to maintain an adequate cardiac index. If it is not, echocardiography can help identifying the causes and verifying the efficacy of corrective maneuvers: It is possible to identify hypovolemia and resolve it, check the correct positioning of the cannulae, and detect masses (especially clots) which can obstruct the venous drainage (Fig. 31.2). In case of low-flow states during ECMO, it is also important to rule out problems to the arterial side of the circuit, for example, aortic dissection (not detected during pre-assistance evaluation or iatrogenic after-cannulation) or severe aortic regurgitation.