VALVAR PULMONARY STENOSIS
Background
Clinical Presentation
In the vast majority of cases, stenosis of the pulmonary valve represents a primary, congenital abnormality. In children, acquired pulmonary valve stenosis is very rare, and typically would occur as a sequela of rheumatic carditis. Regardless of etiology, the clinical presentation of valvar pulmonary stenosis can be variable. In most cases, however, patients are asymptomatic in the face of mild or even moderate pulmonary valve stenosis. With more severe degrees of pulmonary stenosis, symptoms may vary from mild dyspnea and fatigue with exertion to cyanosis and/or overt symptoms of right heart failure. In most cases, the symptoms arise from the decreased ability of the right ventricle to provide cardiac output across the stenotic valve. Signs of heart failure such as low output, hepatomegaly, and edema can be seen in the setting of high central venous pressure due to right ventricular failure in patients with severe pulmonary valve obstruction. Hypoxemia and cyanosis are classic findings in infants with critical pulmonary valve stenosis. Rarely, patients with pulmonary valve stenosis may present with syncope on exertion due to limited right heart output in the face of a severely stenotic valve. As most patients with valvar pulmonary stenosis are asymptomatic, presentation frequently occurs with auscultation of a systolic murmur. A murmur of valvar pulmonary stenosis is typically a systolic ejection murmur, heard best at the upper left sternal border. The murmur often radiates into the lung fields, being frequently more prominent in the left posterior lung field than the right. A murmur is often accompanied by a variable systolic ejection click. Electrocardiographic findings typically feature variable degrees of right axis deviation and the presence of right ventricular hypertrophy.
Anatomy and Physiology
The pulmonary valve is typically thickened and doming with restricted movement in systole. The valve is often bicuspid. In children and young adults, the pulmonary valve annulus size is typically within a normal range and the pulmonary root, sinotubular junction, and main pulmonary artery segments are also often normal in size. Post stenotic dilation of the main pulmonary artery can often be noted. In neonates and young infants, severe pulmonary valve stenosis, along a spectrum of critical pulmonary stenosis, can also be associated with a small pulmonary valve annulus and pulmonary artery segment. In some cases, a very thickened, dysplastic, myxomatous pulmonary valve can be associated with severe stenosis and hypoplasia of the main pulmonary artery segment.
Physiologically, contraction of the right ventricle against a fixed obstruction results in right ventricular systolic hypertension. As a result, variable degrees of right ventricular hypertrophy will develop relative to the degree of outflow tract obstruction. As RV hypertrophy progresses, diastolic dysfunction can develop. Impairment of right ventricular relaxation occurs relatively early during the process of hypertrophy; as the RV thickens it becomes less compliant. RV filling pressures can increase and right atrial pressure may be elevated.
Complications
In mild to moderate pulmonary stenosis, patient symptoms and cardiovascular sequelae may be minimal. In the more severe forms of pulmonary stenosis, chronic right ventricular pressure overload may result in biventricular systolic dysfunction with the development of signs and symptoms of right heart failure. Subsequent dilation of the right ventricle in the face of pressure overload can result in development of tricuspid regurgitation. Chronic right ventricular diastolic dysfunction can result in significant elevations of right heart filling pressures with right atrial dilation. In addition, when atrial level shunts are present, elevation of right atrial pressures can produce right-to-left shunting at the atrial level, producing hypoxemia and cyanosis.
Principles of Echocardiographic Anatomy and Imaging
Two-Dimensional Echocardiographic Anatomy and Hemodynamics
Two-dimensional imaging of the pulmonic valve can be performed in a number of imaging planes. In the parasternal window, the pulmonary valve can be visualized in the long axis by sweeping the plane of sound to the left and anterior from the standard view. In the parasternal short axis, the RV outflow tract and pulmonary valve are typically imaged by sweeping the plane of sound superiorly toward the base of the heart. In the apical four-chamber view, it is often possible to sweep the plane of sound anteriorly to image the subvalvar infundibulum and pulmonary valve, particularly in infants and younger children. The pulmonary valve can also be imaged in the subcostal window in both the coronal and sagittal planes.
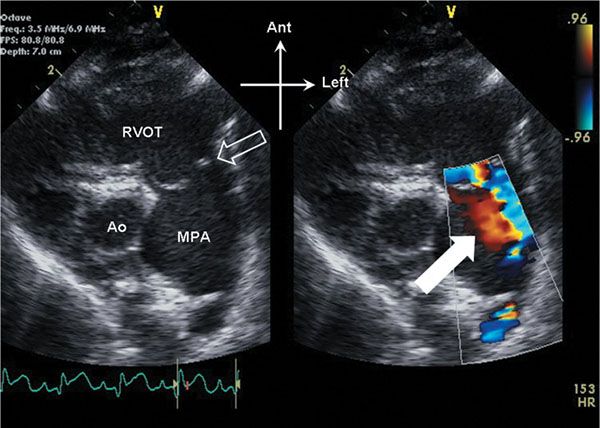
Figure 13.1. Parasternal short-axis view of the right ventricular outflow tract. The pulmonary valve (open arrow) domes in systole. Note the anterior, leftward orientation of the stenotic outflow jet, with swirling of flow (white arrow) seen within the dilated main pulmonary artery (MPA). Ao, aorta; RVOT, right ventricular outflow tract.
On initial inspection, the stenotic pulmonary valve leaflets will appear to be variably thickened. The valve typically domes during systole due to incomplete leaflet opening (Videos 13.1 and 13.2). The pulmonary artery root and main pulmonary artery segment are typically normal in size. In many cases, there can be post stenotic dilation of the main pulmonary artery segment (Fig. 13.1, Video 13.1). The degree of post stenotic dilation in the main pulmonary artery segment does not typically correlate with the degree of pulmonary stenosis and therefore can be seen even in the setting of mild to moderate pulmonary valve stenosis. The subvalvar infundibulum is typically widely patent and normal in caliber, although with significant right ventricular hypertrophy, mild narrowing of the infundibulum may result (Fig. 13.2, Video 13.2).
Careful inspection of the supravalvar portion of the main pulmonary artery in the area of the sinotubular junction should be performed specifically to look for evidence of supravalvar narrowing (Fig. 13.3, Video 13.3). The supravalvar area is typically well seen in the parasternal long- and short-axis windows, but can also be visualized in subcostal windows and on anterior sweeps in the apical window. As the stenotic pulmonary valve typically domes at the level of the sinotubular junction, coexistent supravalvar pulmonary stenosis may be missed on 2D imaging unless this area is examined carefully. Although the pulmonary valve annulus is usually normal in size, it is often useful to measure the pulmonary valve annulus by 2D imaging. Comparison of the annulus dimension to the sinotubular junction will reveal whether the supravalvar area is significantly smaller than the annulus dimension (see Fig. 13.3); the sizes of the two areas are normally about equal. The pulmonary valve annulus is typically measured in either parasternal long- or short-axis views, although the annulus can also sometimes be adequately measured in subcostal views. Although isolated subvalvar pulmonary stenosis is rare, discrete fibromuscular narrowing of the proximal infundibulum (Fig. 13.4) should be distinguished by both 2D and Doppler echo from valvar pulmonary stenosis.
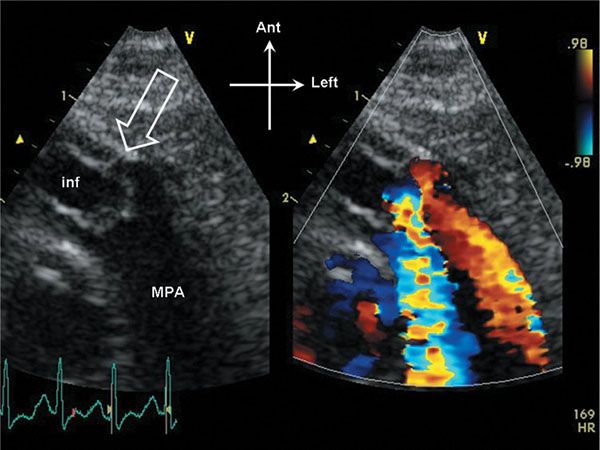
Figure 13.2. Parasternal short-axis view of the right ventricular outflow tract. The pulmonary valve domes in systole, and the annulus dimension is slightly small (open arrow). The infundibulum (inf) is hypertrophied, and appears slightly narrowed in systole. Note that the stenotic flow jet in the main pulmonary artery (MPA) is directed posteriorly in this example.
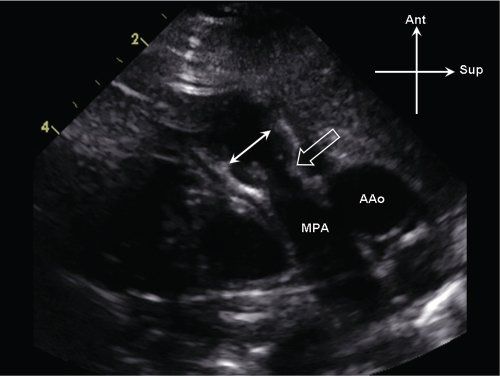
Figure 13.3. Supravalvar pulmonary stenosis. Parasternal long-axis view of the main pulmonary artery (MPA). There is discrete narrowing of the sinotubular junction (open arrow), which is significantly smaller in comparison to the pulmonary valve annulus (white arrows). AAo, ascending aorta.
Hemodynamic assessment is centered on direct estimation of the pressure gradient across the stenotic pulmonary valve, and the sequelae of valve stenosis. Doppler interrogation of the flow across the stenotic pulmonary valve is performed by pulsed-wave and continuous-wave Doppler (Fig. 13.5A). In our experience, accurate alignment of the Doppler interrogation beam with the flow jet is best obtained in either the parasternal short axis or subcostal windows. In the short axis, it will often be evident that the flow jet across the pulmonary valve is often directed more anteriorly than is typical. This somewhat atypical location for the flow jet will often correlate to the area of post stenotic dilation seen in the main pulmonary artery (see Fig. 13.1). The angle of Doppler interrogation should therefore be modified in this instance to better align with the anteriorly directed flow jet. The peak gradient across the stenotic pulmonary valve can be estimated using the modified Bernoulli equation. Color flow Doppler imaging can also allow localization of the gradient to the leaflet tips and also identify any preexisting pulmonary regurgitation due to the anatomic valve abnormality.
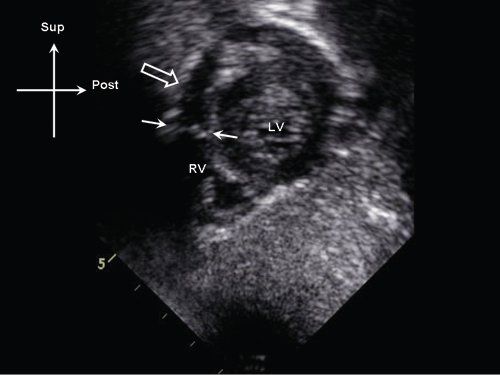
Figure 13.4. Subcostal sagittal view demonstrating a discrete subpulmonary membrane (white arrows) in the proximal right ventricular (RV) outflow tract (white arrows). Note the position of the membrane relative to the pulmonary valve annulus (open arrow). LV, left ventricle.
Variable degrees of right ventricular hypertension will result due to the pulmonary valve obstruction. RV pressure should be estimated, when possible, by measuring tricuspid regurgitant jet velocities using continuous-wave Doppler. Using the peak tricuspid regurgitant jet velocity, the right ventricular to right atrial systolic gradient can be estimated by the modified Bernoulli equation, and by adding an assumed central venous pressure one can solve for estimated right ventricular pressure. Estimates of central venous pressure between 6 and 10 mm Hg are typically used in most labs to perform this quantitation. In the absence of tricuspid regurgitation, indirect evidence of right ventricular hypertension should be assessed. Right ventricular hypertrophy can be quantified as right ventricular anterior wall thickness (by either M-mode or 2D imaging); however, the degree of right ventricular hypertrophy does not correlate well to the gradient across the pulmonary valve, particularly postintervention. Flattening of the interventricular septum can be seen when right ventricular pressure equals the pressure in the left ventricle; when right ventricular pressure is suprasystemic, the interventricular septum will bow leftward into the left ventricle (Fig. 13.6).
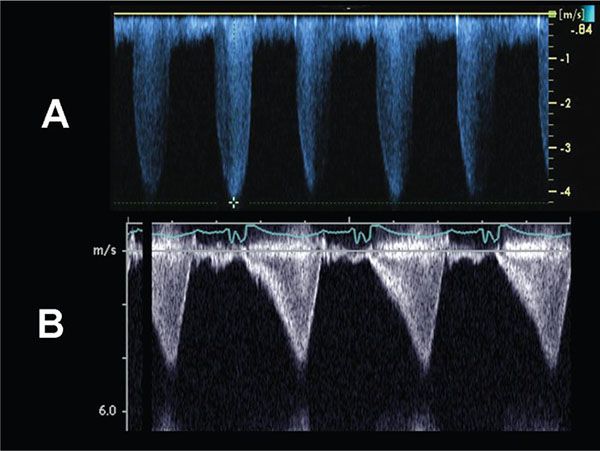
Figure 13.5. Doppler evaluation of pulmonary stenosis. A: The continuous- wave Doppler profile is consistent with fixed obstruction across a stenotic pulmonary valve. The peak instantaneous gradient across the valve in this example = 4(4.25 m/s)2 = 72 mm Hg. B: The continuous-wave Doppler flow profile is consistent with a dynamic obstruction, and predicts a peak instantaneous gradient of ∼70 mm Hg.
Variations on Classic Anatomy
Critical pulmonary stenosis In this group of patients the pulmonary valve typically consists of a thickened, domed, and often dysplastic pulmonary valve (Videos 13.4 and 13.5). In addition, the pulmonary valve annulus is often variably hypoplastic. The degree of pulmonary valvar stenosis is typically severe. The subvalvar infundibulum is often short and muscle-bound due to prominent right ventricular hypertrophy. In addition, the right ventricle is often globally hypertrophied with reduced cavitary size. Critical pulmonary stenosis typically presents in the newborn period with hypoxemia and cyanosis due to the severity of the right ventricular outflow tract obstruction with resultant elevated right heart pressures and right to left shunting at the foramen ovale.
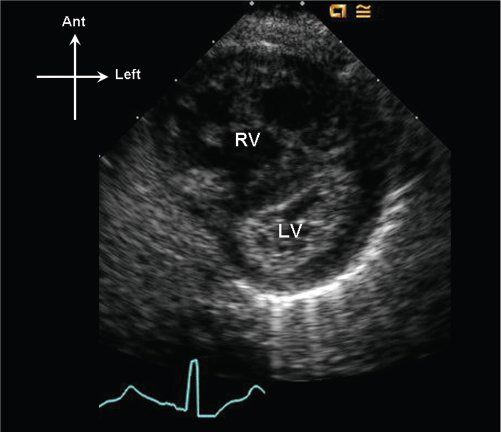
Figure 13.6. Parasternal short-axis image of the right (RV) and left (LV) ventricles. Note that the interventricular septum bows into the LV in late systole, consistent with suprasystemic RV systolic pressure.
Critical pulmonary stenosis belongs to a spectrum of anatomy which includes severe valvar pulmonary stenosis with normal right ventricular size to pulmonary valve atresia with intact ventricular septum and severe right ventricular hypoplasia. Patients with this condition can have variable degrees of pulmonary valve, right ventricular cavity, and tricuspid valve hypoplasia (Fig. 13.7). It is therefore important on a 2-dimensional echocardiographic evaluation to quantify tricuspid valve annular dimension, pulmonary valve annular dimension, and evaluate the degree of RV hypoplasia.
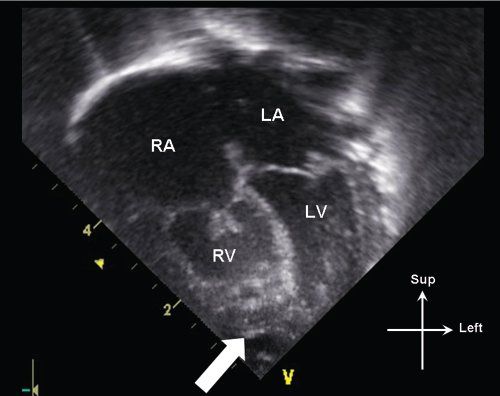
Figure 13.7. Apical 4-chamber view in an infant with critical pulmonary stenosis. Note the mild hypoplasia of the right ventricle (RV), which is not apex-forming (white arrow). The tricuspid valve leaflets are also thickened, with incomplete leaflet coaptation. LA, left atrium; LV, left ventricle; RA, right atrium.
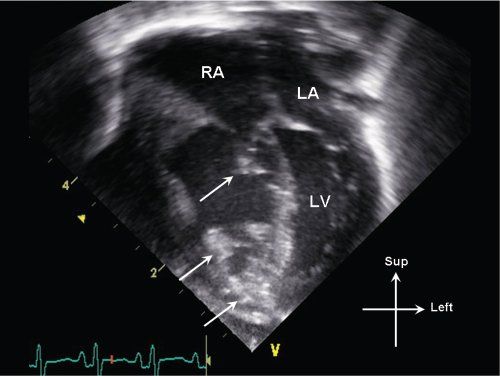
Figure 13.8. Apical 4-chamber view in an infant with critical pulmonary valve stenosis. Note multiple areas of increased echogenicity (arrows) involving the right ventricular endocardium, moderator band, and tricuspid valve apparatus, consistent with endocardial sclerosis. LA, left atrium; LV, left ventricle; RA, right atrium.
In addition to tricuspid valve annular hypoplasia, abnormalities of tricuspid anatomy and function can also be seen. On 2D imaging, severe pulmonary stenosis can be associated with echogenic areas of endocardial sclerosis which may involve the tricuspid valve papillary muscles (Fig. 13.8). The tricuspid valve function can be abnormal with significant degrees of tricuspid regurgitation (Fig. 13.9). The regurgitation may be due in part to anatomic abnormalities involving the tricuspid valve chordal apparatus and papillary muscle function, as well as due to super systemic right ventricular pressure secondary to severe RV outflow tract obstruction. In addition to assessing the severity of tricuspid regurgitation, RV pressure estimates can be obtained using the tricuspid regurgitant jet velocity by applying the Bernoulli equation (see Fig. 13.9).
Additional physiologic information that should be obtained would include the presence or absence of a ductus arteriosus, a pattern of shunting across the foramen ovale (typically right to left), and the presence or absence of a gradient across the pulmonary valve. In a sick neonate, the presence of a widely patent ductus arteriosus and the presence of elevated pulmonary vascular resistance may result in an observed pulmonary valve gradient that is relatively low, and not reflective of the degree of anatomic stenosis (Fig. 13.10). Therefore, demonstration of suprasystemic RV pressure either by tricuspid regurgitant jet velocity (see Fig. 13.9), or 2D evidence of the interventricular septum bowing prominently into the left ventricle in the parasternal short-axis scan (see Fig. 13.6), can often be the most useful physiologic evidence of severity of the pulmonary valve stenosis. In the setting of pulmonary valve atresia, the degree of RV hypoplasia, tricuspid valve hypoplasia, and associated anomalies becomes much higher.
Dysplastic pulmonary valve Occasionally, pulmonary valve stenosis can be associated with severe dysplasia of the pulmonary valve leaflets. This is most typically seen in association with Noonan syndrome. In these patients, the pulmonary valve leaflets are typically very thickened and myxomatous (Fig. 13.11, Video 13.4). Often, it is very difficult to demonstrate normal valve motion on 2D imaging. In addition to the pulmonary valve abnormality, the pulmonary valve annulus is often hypoplastic and the MPA segment beyond the pulmonary valve is also often quite small. Overall, the frequency of pulmonary valve stenosis in Noonan syndrome is approximately 25%, with pulmonary valve dysplasia occurring in approximately 7% of cases (Burch, 1993). Therapeutically, these valves typically do not respond well to balloon valvuloplasty. This is due both to the annular hypoplasia as well as the severe thickening and dysplasia of the pulmonic valve leaflets.
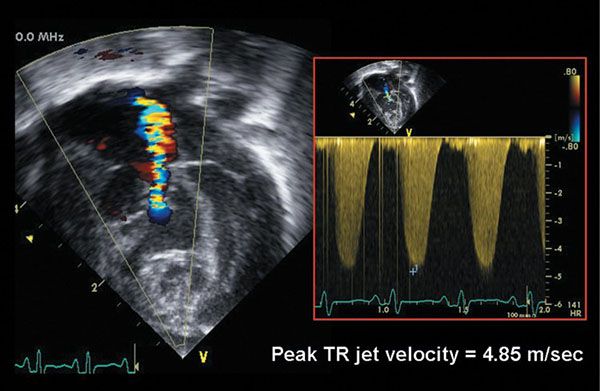
Figure 13.9. Color flow Doppler evaluation of tricuspid valve regurgitation in an infant with critical pulmonary valve stenosis. There is moderate tricuspid regurgitation. Continuous-wave Doppler evaluation of the regurgitant jet velocity (4.85 m/s) predicts a right ventricular pressure-right atrial gradient of 4(4.85)2 = 94 mm Hg. The estimated right ventricular pressure is therefore 94 mm Hg + central venous pressure, or approximately 100–104 mm Hg.
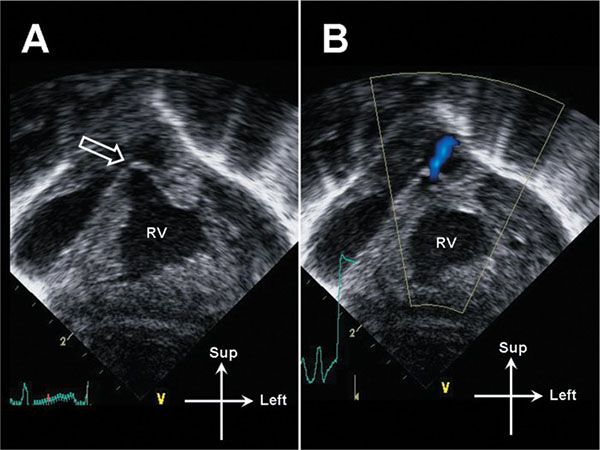
Figure 13.10. Subcostal coronal image of the right ventricle (RV) in a neonate with critical pulmonary valve stenosis. A: The RV is markedly hypertrophied. The pulmonary valve is thickened and doming (open arrow). B: A narrow jet of transvalvar antegrade flow is demonstrated. Note the lack of color flow aliasing; the flow velocity is low due to the presence of a large patent ductus arteriosus (not pictured).
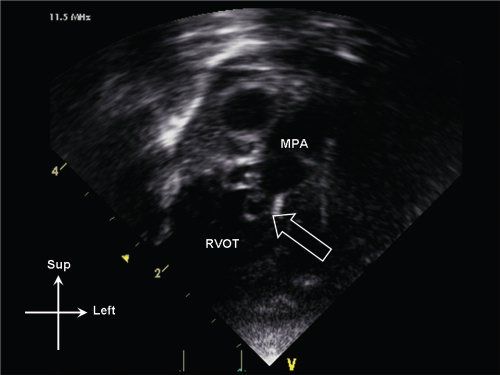
Figure 13.11. Dysplastic pulmonary valve (open arrow) seen on anterior sweep from standard apical window. The valve leaflets are thickened and myxomatous. Note also the poststenotic dilation of the main pulmonary artery (MPA). RVOT, right ventricular outflow tract.
Common Associated Lesions/Findings
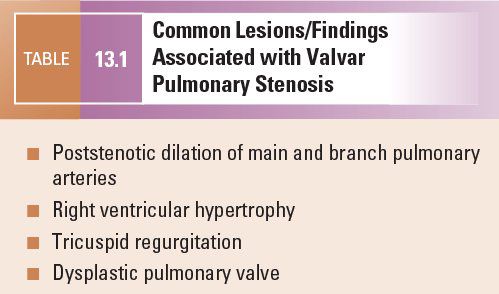
Common lesions associated with valvar pulmonary stenosis are summarized in Table 13.1.
Interventional and Postinterventional Imaging
Cardiac catheterization with balloon valvuloplasty is the treatment of choice when the pulmonary valve annulus is adequate in size and there are no concerns regarding right ventricular or tricuspid valve hypoplasia. Following balloon valvuloplasty, echocardiographic evaluation should be performed to assess for residual pulmonary stenosis gradient, post valvuloplasty regurgitation, and to evaluate right ventricular function and pressure. Occasionally, postintervention subpulmonary stenosis can be observed. In this setting–the so called “suicide right ventricle”–a typical dynamic pattern of obstruction can be observed (see Fig. 13.5B).
Isolated surgical pulmonary valvuloplasty is seldom performed in the current era. However, when the pulmonary valve annulus is hypoplastic, pulmonary valvotomy with a transannular RV outflow tract patch is typically performed to increase the size of the RV outflow tract and relieve valvar obstruction. Following transannular patching, it is typical to see moderate or greater degrees of pulmonary regurgitation, unless the transannular patching was quite limited or the surgical technique included some form of pulmonic valve prosthesis. In the setting of significant pulmonary regurgitation, it is important to follow RV size and function by serial echocardiographic study. Long-standing, significant pulmonary regurgitation can be associated with significant right ventricular dilation and dysfunction.
In the setting of severe pulmonary stenosis or atresia with a hypoplastic right ventricle, single ventricular palliation may be undertaken. Postoperative evaluation of the hypoplastic right ventricle and single ventricular surgical palliation will be discussed in the section on pulmonary atresia with intact septum.
SUBVALVAR PULMONARY STENOSIS
Background
Clinical Presentation
The clinical presentation in patients with subvalvar pulmonary stenosis and intact ventricular septum closely resembles that of isolated valvar pulmonary stenosis. Anomalous muscle bundles in the right ventricular infundibulum, i.e. “double-chambered right ventricle,” are often seen in association with a ventricular septal defect. In this setting, the ventricular septal defect may produce the most prominent clinical findings. As with isolated valvar pulmonary stenosis, when the degree of subvalvar obstruction is mild to moderate, most patients will be asymptomatic. When obstruction is severe and/or long standing, right ventricular hypertrophy and failure may result, with the accompanying clinical findings of right heart failure. On physical examination, the murmur of subvalvar stenosis is typically a long, pansystolic crescendo-decrescendo murmur which is heard at the upper left sternal border. The murmur is often heard lower down the sternal border than is typical in isolated valvar stenosis. Subvalvar pulmonary stenosis is often associated with a palpable thrill along the left sternal border. The pulmonary component of the second heart sound may be more normal than expected—in comparison to valvar pulmonary stenosis—for the degree of murmur on auscultation. A systolic ejection click should not be appreciated. Electrocardiographic findings typically feature variable degrees of right axis deviation and findings of right ventricular hypertrophy, although this will not allow one to distinguish between subvalvar or valvar pulmonary stenosis.
Anatomy and Physiology
The most common form of subvalvar pulmonary stenosis consists of hypertrophied anomalous muscle bundles narrowing the proximal right ventricular infundibulum. This malformation is also known as “double-chambered right ventricle.” Pathologically, the two “chambers” in double chamber right ventricle consist of the right ventricular sinus and inflow portions, which are upstream of the obstructive muscle bundles, and the RV infundibulum and apical trabecular right ventricle, which lay downstream of the obstructive bundles. The muscular bundles themselves appear to consist, most frequently, of hypertrophied septoparietal muscle bands which course anteriorly from the septal band on the ventricular septum to the right ventricular free wall. Some reports have suggested that the muscular obstruction is due abnormal, anterosuperior positioning of the moderator band. It is likely that the nature of the muscular obstruction can vary, and that no single mechanism accounts for all cases. One consistent finding in double-chambered right ventricle is that the downstream chamber includes both the RV infundibulum as well as some portion of the apical trabecular RV. Double-chambered RV is seen frequently in association with a ventricular septal defect, the vast majority of which are perimembranous in location, although muscular and outlet defects have been described. The ventricular septal defect may shunt into either the higher pressure “upstream” RV chamber, or the lower pressure “downstream” chamber, so this should be carefully distinguished by Doppler examination.
Primary fibromuscular infundibular stenoses can also produce subpulmonary obstruction. However, these types of obstruction are significantly rarer than double-chambered right ventricle. In one type, a discreet fibromuscular band separates the main right ventricular cavity from the infundibular chamber. Alternatively, there can be infundibular narrowing due to prominent muscular hypertrophy of the infundibular wall, producing either short or long segment subpulmonary stenosis.
Regardless of the anatomic type of subvalvar stenosis, the physiology is similar. The obstruction will produce elevated pressure in the upstream portion of the right ventricle. As these lesions often consist of a muscular narrowing, there can be a dynamic component with progressive obstruction during ventricular contraction. These findings can be exaggerated during exercise. Right ventricular hypertension is accompanied by a variable degree of compensatory hypertrophy as in other forms of RV outflow tract obstruction.
Complications
Complications relating to right ventricular outflow tract obstruction are indistinguishable from those described for valvar pulmonary stenosis. In mild to moderate outflow tract obstruction, patient symptoms and cardiovascular sequelae may be minimal. When severe, chronic right ventricular pressure overload may result in ventricular systolic dysfunction with the development of signs and symptoms of right heart failure. Right ventricular diastolic dysfunction can result in significant elevations of right heart filling pressures with right atrial dilation.
Principles of Echocardiographic Anatomy and Imaging
Two-Dimensional Echocardiographic Anatomy and Hemodynamics
Subpulmonary obstruction is often most clearly demonstrated in subcostal coronal and sagittal images, particularly in infants and young children. However, subcostal imaging often becomes technically difficult in older children, adolescents, and adults. In the subcostal window, imaging of the right ventricular sinus and the right ventricular outflow tract can be used to demonstrate both double-chambered right ventricle (Fig. 13.12, A–B) as well as the more uncommon subpulmonary ridge or membrane (Figs. 13.13 and 13.14). In double-chambered right ventricle, prominent muscle bundles can be seen traversing the proximal RV outflow tract obliquely on coronal or sagittal imaging (see Fig. 13.12A; Videos 13.6 and 13.7). Color flow Doppler imaging in this area will demonstrate turbulent flow originating at the level of the right ventricular muscle bundles (Fig. 13.15, Videos 13.8 and 13.9)
In the subcostal sagittal view, 2D imaging will again demonstrate the RV muscle bundles in the proximal infundibulum, coursing from the anterior RV free wall to the interventricular septal surface (see Fig. 13.12B, Video 13.7). Imaging of the right ventricular outflow tract in the parasternal long- and short-axis views can often be used to demonstrate subpulmonary obstruction. In double-chambered RV, the parasternal window can also be used to screen for a perimembranous ventricular septal defect often associated with subpulmonary obstruction. In the apical window, particularly in infants and young children, the plane of sound can be swept anterior into the right ventricular outflow tract, and both 2D and color flow Doppler utilized to diagnose and localize subpulmonary stenosis.
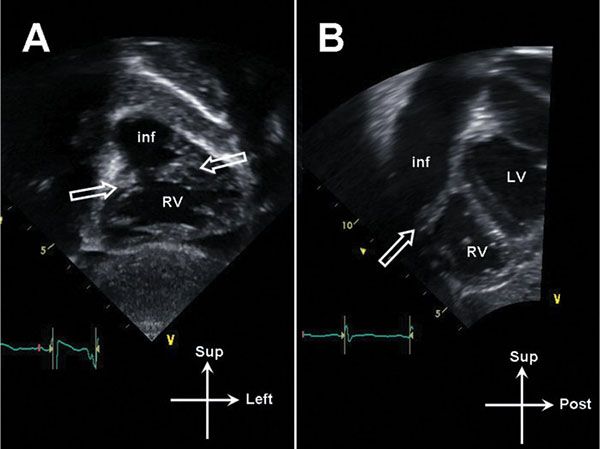
Figure 13.12. Subcostal imaging in double-chambered right ventricle (RV). A: Coronal image demonstrating oblique muscle bundles (open arrows) between the body of the RV and the infundibulum (inf). B: Sagittal image demonstrating muscle bundle (open arrow). LV, left ventricle.
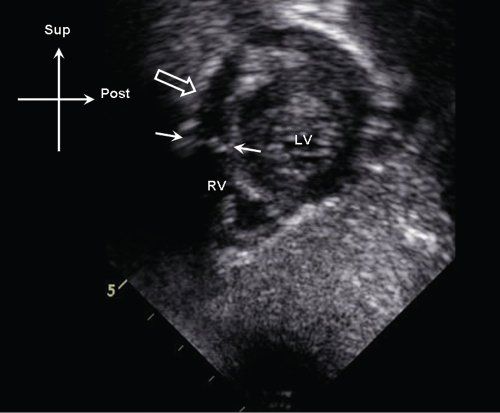
Figure 13.13. Subcostal sagittal image demonstrating discrete membranous obstruction (white arrows) in the proximal right ventricular (RV) outflow tract. Note the position of the membrane relative to the pulmonary valve annulus (open arrow). LV, left ventricle.
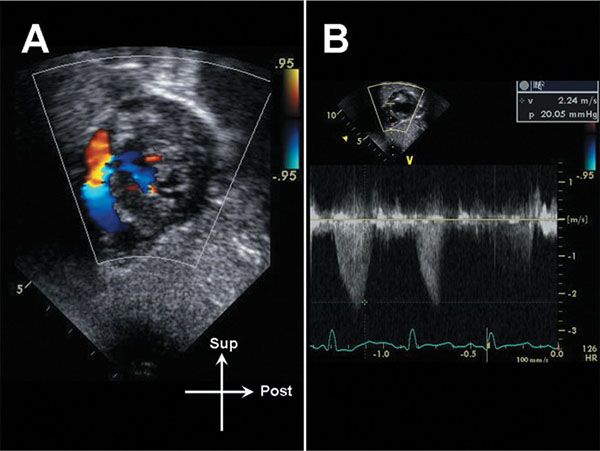
Figure 13.14. Color flow Doppler evaluation of the subpulmonary membrane seen in Figure 13. Note the discrete acceleration at the level of the membrane (A). B: The peak gradient across the membrane is 20 mm Hg.
As double-chambered right ventricle often occurs in the setting of a perimembranous-outlet ventricular septal defect, care must be taken during 2-dimensional imaging to distinguish double-chambered right ventricle with VSD from an anteriorly deviated conal septum in the setting of a tetralogy of Fallot. In cases where the pulmonary valve annulus and distal infundibulum are fairly normal in size, “mild tetralogy” can sometimes be difficult to discern from a double-chambered right ventricle. Lack of aortic valve override of the VSD, normal aortic root size, and prominent hypertrophied RV muscle bundles on the anterior free wall contributing to the obstruction should help distinguish double-chambered right ventricle from tetralogy of Fallot.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


