This chapter details the echocardiographic assessment of congenital mitral valve abnormalities that might be encountered during the evaluation of primary mitral valve pathology or, more frequently, mitral valve pathology in association with other forms of congenital heart disease. It must be stated that congenital mitral valve abnormalities are rare, unlike their rheumatic counterpart. Also, when assessing a patient with suspected mitral valve pathology, it is important to pay attention to associated hemodynamic abnormalities that influence the interpretation of Doppler physiology.
With the recent advent of three-dimensional techniques, our understanding of mitral valve morphology and function as assessed by echocardiography is fundamentally changing. This technique in conjunction with its two-dimensional counterpart is currently becoming the reference standard for surgical or medical intervention.
This chapter will address both the two- and three-dimensional approach to the assessment of mitral valve abnormalities and their hemodynamic consequence.
MITRAL VALVE DEVELOPMENT
The atrioventricular junction comes into prominence following rightward looping of the heart tube after the 25th day of gestation. By the end of the fifth week, the developing ventricles are visible, with the future left ventricle supporting a large portion of the atrioventricular canal. The lumen of the atrioventricular canal is occupied by the inferior and superior endocardial cushions. Initially unfused, these cushions eventually fuse during the sixth week and form the right and left atrioventricular junctions, to which the developing leaflets of the mitral valve will be anchored. Parts of these fused cushions remain on the left side of the septal crest and form the aortic leaflet, often referred to as the anterior leaflet, of the mitral valve.
Formation of the normal mitral valve can only proceed when the aorta becomes committed to the left ventricle, resulting in fibrous continuity between the two leaflets; hence the name the aortic leaflet is given, which distinguishes it from its mural counterpart, often referred to as the posterior leaflet. Initially, there is still a cleft at the parietal margins of the fusion of the superior and inferior cushions. The mural leaflet of the mitral valve develops from the lateral cushion tissue of the atrioventricular canal myocardium that protrudes into the ventricular lumen. The myocardium disappears via apoptosis, and therefore the entire leaflets are derived from cushion mesenchyme that is endocardial in origin. This provides an explanation as to why persistence of the myocardium results in the mitral arcade. Although the tricuspid septal leaflet delaminates from the ventricular myocardium, the aortic leaflet is never attached to or supported by the myocardium, except at its cranial and caudal margins, which represent chordal and papillary muscle attachments. Expansion of the inferior quadrants of the left atrioventricular junction involves growth of the parietal wall of the left ventricle, with comparable growth of the lateral cushion. This eventually results in the lateral cushion occupying two-thirds of the circumference of the developing mitral valve.
This expanded crescent, which represents the developing mitral valve, is associated with compacting columns in the spongy layer of the ventricular muscle, which eventually form the papillary muscles. Excessive or abnormal compaction of the trabecular layer of the developing ventricular myocardium is responsible for the parachute mitral valve. Failure of the formation of the tendinous chords from the myocardial primordium results in the mitral arcade lesion, with muscle extending from the tips of the leaflets to the papillary muscles. When Ebstein’s malformation of the mitral valve occurs, it is the mural leaflet that is involved, as this is the leaflet that excavates from the parietal ventricular wall.
GENETICS OF MITRAL VALVE DEVELOPMENT
Reciprocal signaling between the endocardial and myocardial cell layers in the cushion is mediated in part by transforming growth factor (TGF) family members and induces a transformation of endocardial cells into mesenchymal cells. This is induced by BMP-2 signals that are derived from adjacent myocardial cells. Sox9 is activated when myocardial cells undergo mesenchymal transformation and Sox9-deficient mesenchymal cells fail to express ErbB3, which is required for cushion cell proliferation. The mesenchymal cells migrate into the cushions and differentiate into the fibrous tissue of the valves. Several genes play a role in heart valve formation, including calcineurin with signaling and downstream activation of the NFAT (nuclear factor of activated T cells) family of transcription factors, with an absence of these resulting in fatal defects of valve formation.
ANATOMY OF THE MITRAL VALVE
About two-thirds of the annulus of the mitral valve is supported by a fibroareolar junction, which serves also to separate the parietal portion of the left atrial myocardium from the ventricular myocardium. The remaining third of the ring is part of an extensive sheet of fibrous continuity with the leaflets of the aortic valve, strengthened at its ends by the left and right fibrous trigones. There are two so-called commissural areas within the valve, corresponding more or less to the areas of the fibrous trigones. The zone of apposition between these areas delineates the two primary valvar leaflets. The more extensive leaflet is attached to the parietal part of the annulus. It is more accurate to describe this as the mural leaflet, rather than posterior. It has relatively little depth; consequently, when the valve is closed, it is seen as a long rectangular structure that is usually divided into scallops. There are usually three scallops, with a large central and then smaller lateral and medial structures. The second major leaflet of the valve is attached along the area of aortomitral fibrous continuity. Although its overall shape is semicircular, it is much squarer and more boxlike than the long, rectangular mural leaflet. Often termed the “anterior” leaflet, it is not strictly anterior in position. That is why we prefer to describe it as the aortic leaflet.
The tension apparatus of the valve consists of the tendinous cords and the papillary muscles. The important cords are those supporting the free edge of the leaflets (notably the commissural fan-shaped cords) and those supporting the rough zone (particularly the thick strut cords) of the aortic leaflet and the basal cords. The cords supporting the free edge are much more significant in support of the mural leaflet. In contrast, the rough zone and strut cords are more significant in support of the aortic leaflet. A study by Becker and de Wit showed considerable variation of the free edge chords in normal hearts. The papillary muscles of the mitral valve are relatively constant in position, although they, too, show marked variation in their detailed anatomy. They are sited beneath the ends of the zone of apposition between the leaflets in posteromedial and anterolateral position and have a typical paired appearance. The axis of opening of the valve subtends a considerable angle relative to the inlet septum. Unlike the septal leaflet of the tricuspid valve, the leaflets are never attached by tendinous cords directly to the inlet component of the muscular ventricular septum.
Mitral Annular Shape and Function
The shape of the mitral annulus was initially thought to be planar, which resulted in a gross overdiagnosis of mitral valve prolapse. With the advent of three-dimensional echocardiography, this was recognized as being incorrect, as the mitral annulus has both high and low points, appearing with a shape like a saddle (Figs. 9.1 and 9.2). The anterior and posterior aspects represent the high points, while the medial and lateral represent the low points. Therefore, unless severe, mitral valve prolapse should be confidently diagnosed only in the long-axis view.
The saddle shape of the valve is most likely responsible for reducing leaflet stress, which is important when designing prosthetic valve rings or performing other surgical procedures that impact normal annular function.
Previous animal and human studies demonstrated that mitral annular motion is very heterogeneous. The reported expansion of anterior mitral annulus and aortic root during ventricular systole corresponds with segmental diameter changes observed from previous data. The commissure-to-commissure (C–C) direction of the mitral annulus, which is muscular on both sides, moves dynamically throughout the cardiac cycle; however, the anteroposterior direction moves differently due to the sites of fibrous continuity with the aortic valve. In early systole, the mitral annular segments in the C–C direction start to contract, followed by diameter reduction in the anteroposterior direction with a slight expansion in the C–C direction in mid-systole, which continues on during late systole. This shape change is thought to permit maximal expansion of the aortic root to facilitate ejection and good coaptation of the mitral valve leaflets.
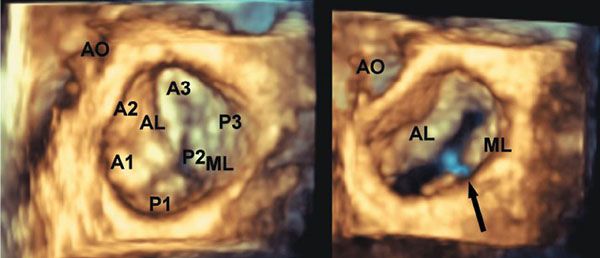
Figure 9.1. Three-dimensional image of the mitral valve as seen from the left atrium. Note the annular shape. The aortic and mural leaflets can be seen. Also, note the nomenclature used for assessing the individual components of the leaflets (A1–A3 for the aortic or anterior leaflet, and P1–P3 for the posterior or mural leaflet). AO, aorta; AL, aortic leaflet; ML, mural leaflet.
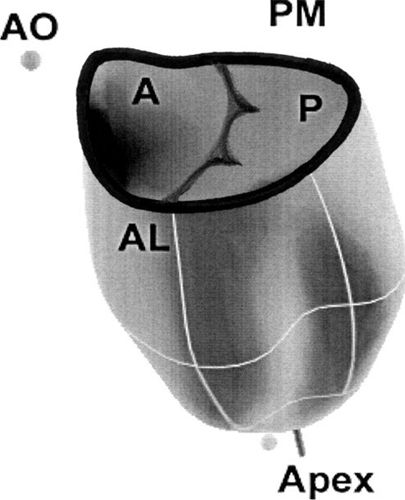
Figure 9.2. Mitral annular shape in the normal heart. Note the high points at the anterior and posterior parts of the annulus. The maximum bending of the annulus occurs along the commissures between the aortic and mural leaflets. A, anterior; AL, anterolateral; AO, aortic leaflet; P, posterior; PM, posteromedial.
Left atrial contraction contributes to area reduction of the mitral annulus before the ejection phase, contributing to 89% of area reduction in adult sheep. The contribution of atrial contraction to annular diameter reduction is most prominent in the anterolateral–posteromedial direction (i.e., the C–C direction for mitral valve) in children. This result was different from data in adult sheep for the mitral annulus, which showed a more prominent diameter reduction in the anteroposterior direction during atrial contraction. This difference might be due to the difference in species, the maturity of myocardial tissue, and the fact that the human control subjects were not under general anesthesia.
When measured as C–C, the mitral annulus starts to bend in mid-systole, becoming most acute during the isovolumic relaxation phase of the mitral annulus (Fig. 9.3). After this, the annulus flattens, which may have a role in early diastolic filling. There appears to be a positive correlation between the bending angle of the mitral annulus and global left ventricular systolic function, which corresponds with the finding that the mitral annulus becomes planar after myocardial ischemia.
Mitral Valve Leaflets
It is generally accepted that the mitral leaflets move closer together, after opening widely at the beginning of the E wave, with leaflet separation being maximal at the second left atrial/left ventricular pressure crossover, a pattern that corresponds exactly to the E wave as described in the use of Doppler echocardiography.
The line of closure of the mitral valve is on the atrial aspect of the valve and is about one-third of the distance from the free edge of the leaflets to their annular attachment. It is important to note, therefore, that the valve does not close at its free edge. This aspect of valve closure is much better viewed by three- rather than two-dimensional echocardiography (see Fig. 9.1).
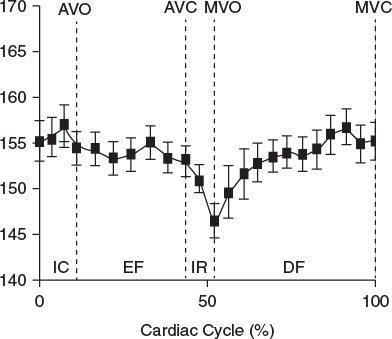
Figure 9.3. Graph demonstrating the bending angle in the normal mitral annulus in a pediatric case. Note the annulus starts to bend in late systole, becoming maximal during isovolumic relaxation, and subsequently becomes flatter. AVO, aortic valve opening; AVC, aortic valve closure; DF, diastolic phase; EF, ejection phase; MVC, mitral valve closure; IC, isovolumic contraction; IR, isovolumic relaxation; MVO, mitral valve opening.
Papillary Muscle Location
It is well accepted that in patients with ischemic mitral regurgitation, leaflet tethering due to papillary muscle displacement is responsible for restricted diastolic excursion that is independent of inflow volume. In addition, this mechanism is responsible for poor systolic coaptation and the ensuing regurgitation. Therefore, there is an important and definite relationship between the mitral valve papillary muscles, leaflets, and supporting chordae to maintain normal valve function. Three-dimensional echocardiography provides a unique opportunity to image these relationships (Fig. 9.4), providing views that cannot be obtained with two-dimensional echocardiography.
It is also possible to see the chordal apparatus in three dimensions, which may have future implications with regard to their importance in mitral valve tethering (Fig. 9.5). More intriguing is the finding that the mitral aortic leaflet is able to adapt to increased mechanical stress by expanding, with associated thickening and lengthening of the chordal apparatus. The histological changes noted were those of altered endothelial protein expression, which suggests possible reactivation of embryonic developmental pathways.
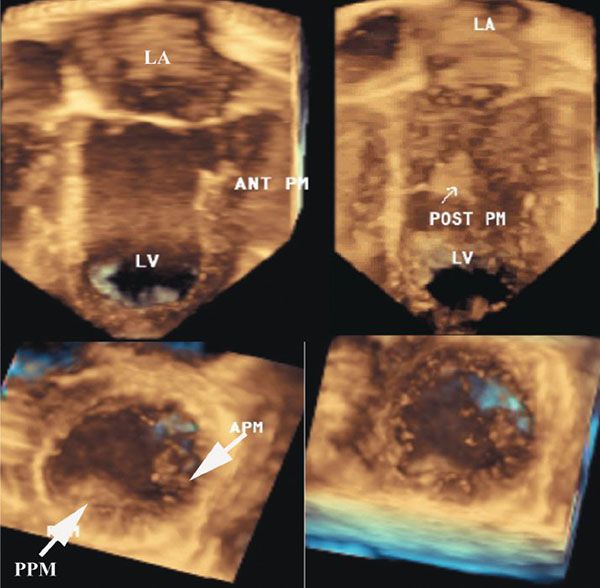
Figure 9.4. Three-dimensional image of a normal mitral valve. Top left: Aortic leaflet, which is inserted into the anterior papillary muscle. Top right: Posterior papillary muscle. Bottom left: Two papillary muscles. Bottom right: Apex of the left ventricle. APM, anterior papillary muscle; LA, left atrium; LV, left ventricle; PPM, posterior papillary muscle.
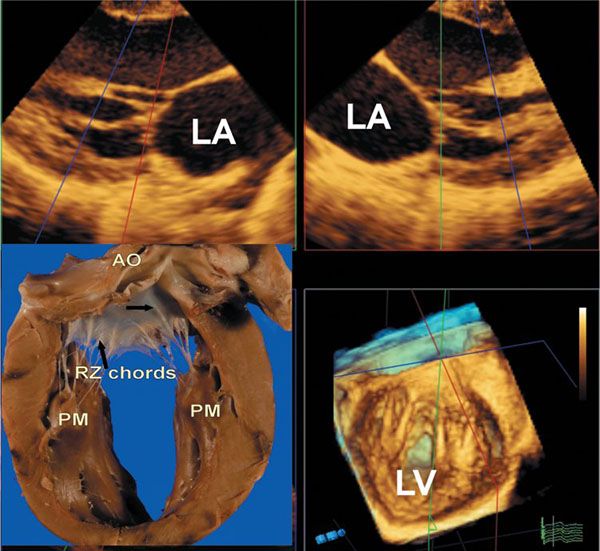
Figure 9.5. Three-dimensional images of the mitral chordal apparatus. The upper two images are from the same full volume data set seen in the lower right-hand panel. These demonstrate two-dimensional images of specific chordal components of the mitral valve. The lower right-hand panel shows the chords inserting onto the aortic leaflet, as well as the supporting two papillary muscles. The lower left-hand panel is a specimen which shows a strut chord as indicated by the upper right-hand arrow, and rough zone chords by the second lower black arrow. AO, aorta; LA, left atrium; LV, left ventricle; PM, papillary muscles; RZ, rough zone.
INCIDENCE OF CONGENITAL MITRAL VALVE ABNORMALITIES
Congenital deformities of the mitral valve are rare, with mitral stenosis occurring in 0.6% of postmortem studies and in 0.21% to 0.42% of clinical series. Congenital mitral incompetence is rarer. There is a male-to-female ratio of around 1.5:1 to 2.2:1. The fully developed syndrome of “Shone syndrome” includes four obstructions within the left heart: the valvar lesion itself, supravalvar mitral ring, subaortic stenosis, and aortic coarctation. Any of these obstructions may coexist with any congenital lesion afflicting the mitral valve, particularly coarctation. Annular hypoplasia of the mitral valve is almost always associated with hypoplasia of the left ventricle and aortic stenosis or atresia. This may also be seen in association with ventricular septal defect or double-outlet right ventricle and tetralogy of Fallot. When the mitral valve is imperforate, left ventricular hypoplasia is inevitable unless there is an associated ventricular septal defect.
SPECIFIC MITRAL VALVE PATHOLOGY
At the outset, it should be stated that although in some cases there is pure mitral valve stenosis or regurgitation, in many other cases there is a combination.
Mitral Valve Dysplasia and Hypoplasia
With mitral valve dysplasia, the leaflets are thickened, the interchordal spaces often are obliterated, and the papillary muscles are deformed, the last frequently extending as muscular strands directly into the leaflets. Usually such a valve shows global hypoplasia and is the most common lesion associated with isolated congenital mitral stenosis. When the free edges of the dysplastic leaflets are thickened and rolled, the valve may be incompetent as well as stenotic. This appearance can be viewed in the parasternal long-axis, short-axis, and four-chamber views by two-dimensional echocardiography, with evidence of thickened leaflets and reduced mobility (Fig. 9.6A). Of note, it is often difficult to differentiate between the edge of the leaflets, the chordal apparatus, and the papillary muscles.
Color flow Doppler demonstrates the flow acceleration just proximal to the mitral annulus, followed by variance as the blood exits the restrictive orifice. In many cases, there are multiple exits due to the fused nature of the chordal apparatus (Fig. 9.7). In some cases, there is laminar flow through the orifice into the center of the valve, with more distal exits (Fig. 9.8).
Three-dimensional echocardiography provides an en face view of the mitral valve. The valve can be seen from the left ventricular aspect, providing full anatomical details of the valve. The advantage of the “looking up” view from the left ventricular cavity is that exquisite detail regarding the commissures can be viewed (Fig. 9.6B). Although the surgical en face view is good, it is often difficult to appreciate the commissural detail due to the normal curved closure line of the mitral valve. To overcome this, it is possible to provide a slightly lower cut plane, which allows visualization of the commissures as well as the valve-supporting apparatus. It is also possible to remove the front of the left ventricle, such that the anterior aspect of the mitral valve and its tension apparatus can be seen. Three-dimensional color flow Doppler remains at a somewhat primitive stage and at present does not provide much added diagnostic information in congenital mitral valve stenosis (Fig. 9.7). Part of the problem is that, unlike rheumatic mitral valve stenosis, there are frequently multiple small eccentric jets, which make vena contracta summation difficult (see Fig. 9.8).
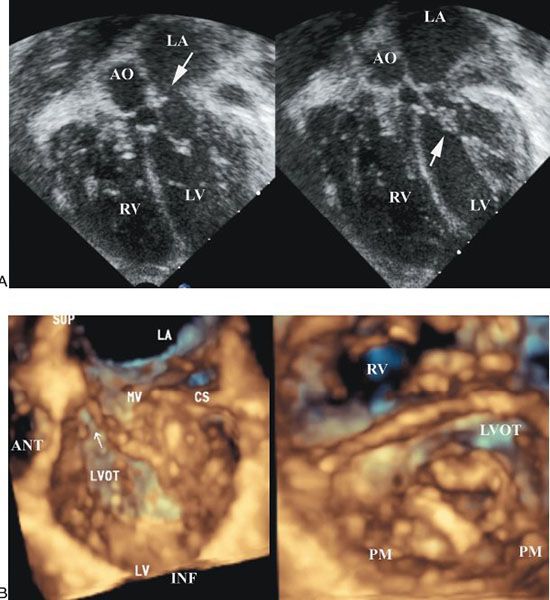
Figure 9.6. Patient with dysplastic mitral valve stenosis. A. Two-dimensional four-chamber view showing a small associated mitral valve ring (left) and the thickened mitral leaflets and subvalvar apparatus (right). AO, aorta; LA, left atrium; LV, left ventricle; RV, right ventricle. B. Three-dimensional echocardiogram from the same patient as in A. Left: Images from above the mitral valve. The supramitral ring is well seen. Right: Thickened mitral valve leaflets as well as tethering of the mural leaflet. ANT, anterior; CS, coronary sinus; INF, inferior; LA, left atrium; LVOT, left ventricular outflow tract; MV, mitral valve; PM, papillary muscle; RV, right ventricle; SUP, superior.
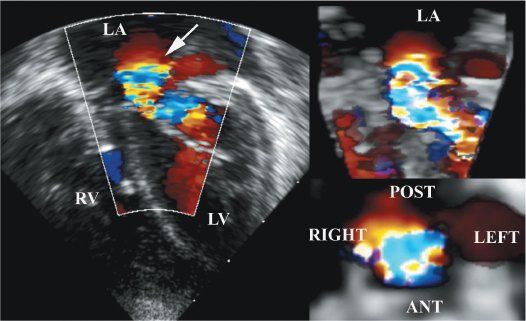
Figure 9.7. Color flow Doppler in the same patient as Figure 9.6. The two-dimensional color flow Doppler shows that the acceleration is occurring just above the annulus, which is consistent with the supramitral ring. Note the narrow inflow orifice. Upper right: Three-dimensional color flow data. Bottom right: En face view of the stenotic jet. LA, left atrium; LV, left ventricle; RV, right ventricle.
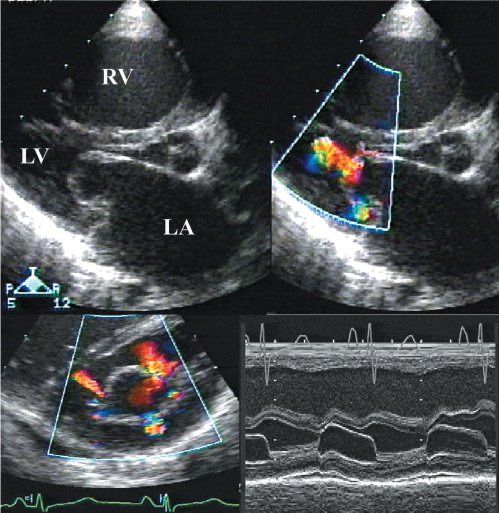
Figure 9.8. Mitral valve stenosis with multiple exit points and laminar flow centrally. Note in both the short- and long-axis views, there is no flow acceleration centrally. The M-mode shows a prolonged E-F slope. LA, left atrium; LV, left ventricle; RV, right ventricle.
Occasionally, mitral stenosis may occur when the valve is miniaturized in its entirety but does not show dysplastic features. Of more significance is the arrangement when only part of the valve is hypoplastic. This is typically seen when one papillary muscle, usually the anterolateral muscle, is grossly reduced in size or even totally absent. Then the anterolateral commissure either inserts directly on to the left ventricular free wall or is supported on the wall by a small papillary muscle (Fig. 9.9).
The tension apparatus then has a grossly eccentric appearance, effectively inserting into a solitary papillary muscle. This arrangement was illustrated by Shone and colleagues as a “parachute mitral valve.” This is different from those cases where the two papillary muscles are fused into a solid solitary structure that supports the tension apparatus from the entire valve. In addition, an echocardiographic/morphologic correlation noted that many patients with aortic coarctation have altered positions of the left ventricular papillary muscles and narrowing of the interpapillary valley.
A supravalvar stenosing ring was described by Shone and his colleagues as part of the complex including the parachute valve. The supravalvar ring in this setting was a concentric thickening of the left atrial endocardium immediately above the atrioventricular junction. In the clinical setting, in contrast, it seems that the so-called stenosing supravalvar ring is formed on the atrial aspect of the valvar leaflets (Fig. 9.10). This is best seen in the parasternal long-axis view by two-dimensional echocardiography, as the beam is perpendicular to the ring in the axial plane where the resolution is best. Although it can be imaged in the four-chamber view, depth and lateral resolution may make differentiation from the annulus and leaflets difficult. Color flow Doppler is very helpful in this setting, as the flow acceleration seen proximal to the stenosis occurs well above the mitral annulus, with the variance seen at the annular level (see Fig. 9.7).
One of the advantages of three-dimensional echocardiography is that the ring can be seen in its entirety, unlike that seen by two-dimensional echocardiography. In the majority of cases the supravalvar ring does not occur in isolation but is seen in association with valve dysplasia. In some instances, there is the appearance of a membrane-like structure below the annulus that traverses the orifice of the valve. Whether this represents a component of valve dysplasia or is related to the supravalvar ring is unclear.
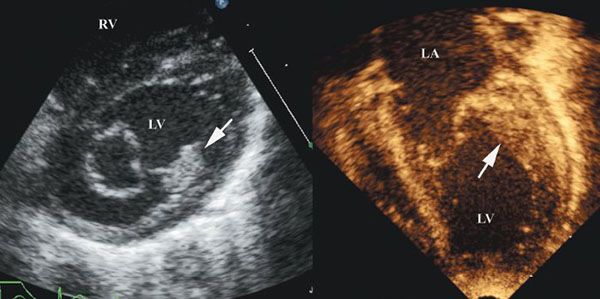
Figure 9.9. Two-dimensional image in a patient with a dominant anterior papillary muscle. LA, left atrium; LV, left ventricle; RV, right ventricle.
Anomalies of the Mitral Valve Leaflets
The most extreme anomaly is an imperforate mitral valve. This usually coexists with aortic atresia, forming part of the “hypoplastic left heart syndrome.” An imperforate mitral valve can also be found without aortic atresia and is then part of the combination termed “mitral atresia with patent aortic root.” Often, the ventriculoarterial connection is double-outlet right ventricle; however, in a significant number of cases, the patent aorta arises from a good-sized left ventricle that is connected via a ventricular septal defect. The ventricular septal defect can be of varying morphology.
Ebstein’s malformation can rarely affect the morphologically mitral valve. The characteristic feature of Ebstein’s malformation of the mitral valve is that the mural leaflet is plastered down onto the ventricular wall; consequently, its hinge is below the atrioventricular junction but there is no thinning of the atrialized inlet portion as is usually seen when it is the morphologically tricuspid valve that is involved.
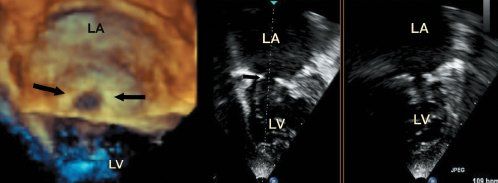
Figure 9.10. Two- and three-dimensional images from a patient with a supramitral ring. The left-hand panel is a three-dimensional image of the complete supramitral ring, as seen from the left atrial view and outlined by the black arrows. The middle and right-hand panel show parts of the ring as seen by two-dimensional echo. LA, left atrium; LV, left ventricle.
An isolated cleft of the mitral valve is also an anomaly confined to the leaflet, and one that primarily produces mitral incompetence. The affected leaflet tends to be dysplastic, and its edges are usually rolled and thickened. It is important to distinguish an isolated cleft of the aortic leaflet of the mitral valve in hearts with a separate atrioventricular junction from a “cleft” in the left valve of atrioventricular septal defects with a common atrioventricular junction. The isolated cleft “points” into the aortic outflow tract, often in association with a ventricular septal defect, and the aortic leaflet is readily reconstituted by suture of its edges (Fig. 9.11).
In contrast, the so-called cleft in atrioventricular septal defects points to the septum and represents the space between the bridging leaflets. Closure of the bridging leaflets cannot produce a left atrioventricular valve that in any way resembles a normal mitral valve. These features, as well as differentiation from the cleft of an atrioventricular septal defect, are well viewed by both two- and three-dimensional echocardiography. One advantage of the latter technique is that the precise length of the cleft can be viewed and measured (Figs. 9.12 and 9.13).
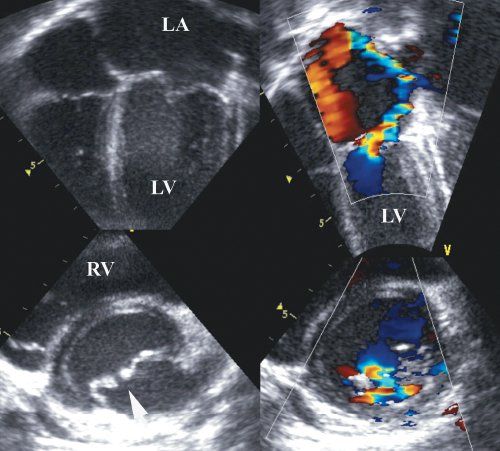
Figure 9.11. Isolated cleft of the mitral valve with significant regurgitation. Note the edges of the cleft are thickened and unsupported. LA, left atrium; LV, left ventricle; RV, right ventricle.
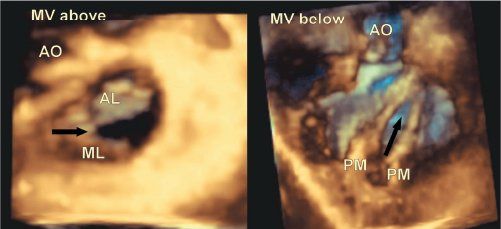
Figure 9.12. Isolated cleft of the mitral valve in a patient who had associated transposition of the great arteries and a ventricular septal defect. The left-hand panel is from the left atrial view, and the right-hand one from the left ventricular aspect. Note that in this case the cleft (black arrow) is eccentric, which is common in this setting. AO, aorta; AL, aortic leaflet; ML, mural leaflet; MV, mitral valve; PM, papillary muscle.
In addition, the location and extent of the regurgitation are better viewed with three-dimensional echocardiography. A significant problem with imaging color flow jets by two-dimensional echocardiography is that it is frequently difficult to appreciate multiple jets when imaging in one plane. Jets change direction, which provides additional confusion when changing from one imaging plane to another. Likewise, the presence of central and lateral commissural jets is best seen with three-dimensional echocardiography (Fig. 9.14).
Double-Orifice Mitral Valve
The most common anatomical presentation is that in which a tongue of valvar tissue extends between the mural and aortic leaflets, dividing the valvar orifice into two components. Such an arrangement is frequently encountered in the left valve seen in an atrioventricular septal defect. The rarer variant, involving the otherwise normal mitral valve, shows duplication of the entire valvar structure. As a result, the left atrium is connected to the left ventricle by two valves, each with its own annulus, leaflets, cords, and papillary muscles (Fig. 9.15). This anomaly can exist in otherwise normal hearts where it is often discovered coincidentally, as well as in more complex anomalies such as tricuspid atresia or double-inlet ventricle.
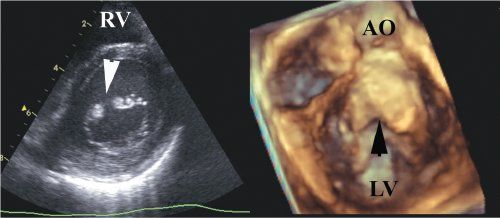
Figure 9.13.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


