The most common congenital malformations involving the tricuspid valve are Ebstein’s malformation and tricuspid valvar dysplasia. This chapter will primarily consider Ebstein’s malformation with concordant atrioventricular connections and biventricular circulation. Ebstein-like deformation of the morphologically tricuspid valve in association with atrioventricular discordance is discussed in Chapter 10. Isolated dysplasia of the tricuspid valvar leaflets, traumatic tricuspid regurgitation, and other right ventricular abnormalities will be briefly described, and the differences between these disorders and Ebstein’s malformation are highlighted. Echocardiography has become the procedure of choice for both the diagnosis and long-term assessment of patients with Ebstein’s malformation. In the 1980s, cross-sectional echocardiography replaced M-mode as the clinical standard. As early as 1984, cross-sectional imaging was considered sufficiently comprehensive that angiography was no longer necessary to diagnose Ebstein’s malformation.
ON THE NAMING OF THE TRICUSPID VALVAR APPARATUS
We have chosen to adopt an anatomically unambiguous system of names to describe the components of the tricuspid valvar apparatus. This will create some differences between the descriptions contained within this chapter and in many previous publications. It seems best to concretely define this system to avoid potential confusion. The three valvar leaflets will be referred to as the anterior, the septal, and the inferior leaflets (Fig. 8.1). The designations of the anterior and septal leaflets are clear. The inferior leaflet has often been called the “posterior” leaflet. This designation is anatomically incorrect. This leaflet is positioned inferiorly within the ventricular cavity, lying adjacent to the diaphragm in the normal heart. We recognize that abnormal valves often display rotation of their components away from the normal position. However, the inferior tricuspid leaflet is always associated with the ventricular free wall and is never in a posterior position. The posterior aspect of the right ventricle is the interventricular septum, not the free wall adjacent to the diaphragm. Therefore, in the human heart, it would be inappropriate to refer to the tricuspid valve leaflet nearest the free wall as being “posterior,” when it is not. This leaflet is always positioned inferior to the anterior and septal leaflet, even in the most severely distorted valves, leading to our preference for the name “inferior” leaflet of the tricuspid valve.
Ebstein’s Malformation
Ebstein’s malformation has an extremely variable natural history. The clinical course depends on the degree of abnormality manifested by the right ventricle and the tricuspid valvar apparatus. These abnormalities can range from minimal to severe. If the deformity of the tricuspid valve is severe, it may result in profound congestive heart failure in the neonatal period, or even in intrauterine death. At the other end of the spectrum, patients with a mild degree of displacement and dysfunction may remain asymptomatic until late adult life.
DEFINITION AND ANATOMIC FEATURES OF EBSTEIN’S MALFORMATION
Ebstein’s malformation is often thought of as a primary disorder of the tricuspid valve. In reality, it is a manifestation of a more global aberration in myocardial development. Those afflicted with Ebstein’s malformation invariably display abnormalities in both myocardial structure and function, as well as the characteristic valvar deformities. The right ventricle and tricuspid valve are universally involved, while changes in the left heart are less common.
The hallmark of Ebstein’s malformation is displacement of the annular attachments (hinges) of the septal and inferior leaflets, away from the atrioventricular junction (Fig. 8.2). This results from failure of these leaflets fully to separate from the underlying ventricular wall during cardiac development. The normal separation process is referred to as “delamination” (Fig. 8.3). Delamination begins at the tips of the embryonic leaflets and proceeds “back” toward the atrioventricular junction. A completely delaminated leaflet will have a hinge point at (or very near) the anatomic tricuspid valvar annulus.
This failure of delamination results in the leaflets remaining variably adherent to the underlying right ventricular and septal myocardium (Fig. 8.4). This adherence creates the characteristic displacement of the annular attachments of the valve and rotates its functional orifice away from the normal position within the right ventricular inlet. It is now appreciated that the concept of apical displacement of the tricuspid valve in Ebstein’s malformation was not anatomically precise. The displacement seen in Ebstein’s malformation is not simply a linear shift of the tricuspid valve toward the cardiac apex. The displacement is actually rotational or spiral in nature, following the contours of the right ventricular cavity. The primary orientation of the rotation is in an anterior direction, toward the right ventricular outflow tract, and only to a lesser extent toward the apex. This spiral shift in the tricuspid valve apparatus moves the functional valvar orifice to the junction of the trabecular and outlet zones of the ventricle (Figs. 8.5 and 8.6). In the most severe cases, the functional leaflets may be positioned within the outlet itself. The adherent portions of the valvar leaflets usually have little or no motion. This typically leads to tricuspid regurgitation or, more rarely, to stenosis.
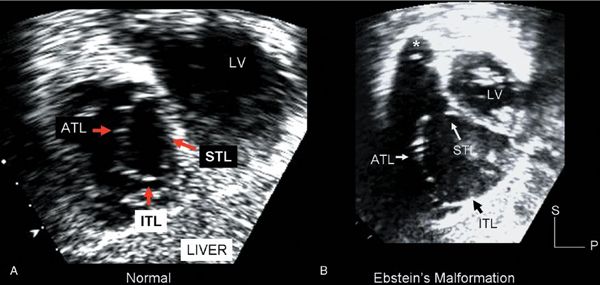
Figure 8.1. These subcostal, sagittal plane images demonstrate the anatomic relationships of the three tricuspid valve leaflets. A: Normal tricuspid valve. The septal tricuspid leaflet (STL) lies parallel to the ventricular septum, and the anterior tricuspid leaflet (ATL) is positioned parallel to the anterior free wall of the right ventricle and separates the posterior inlet from the anterior outlet of the ventricle. The inferior tricuspid leaflet (ITL) is parallel to the diaphragmatic surface of the right ventricle and lies in the most inferior portion of the ventricle. This leaflet has often been referred to as the “posterior” leaflet, which is anatomically incorrect. B: From an examination of the patient with Ebstein’s malformation. The ATL remains parallel to the anterior free wall. The STL and ITL are more difficult to recognize in this diastolic image because they are adherent to the myocardium of the septum and right ventricular inferior wall. Only a small segment of the STL is seen separated from the ventricular septum near the right ventricular outflow tract (arrow). LV, left ventricle; P, posterior; S, superior.
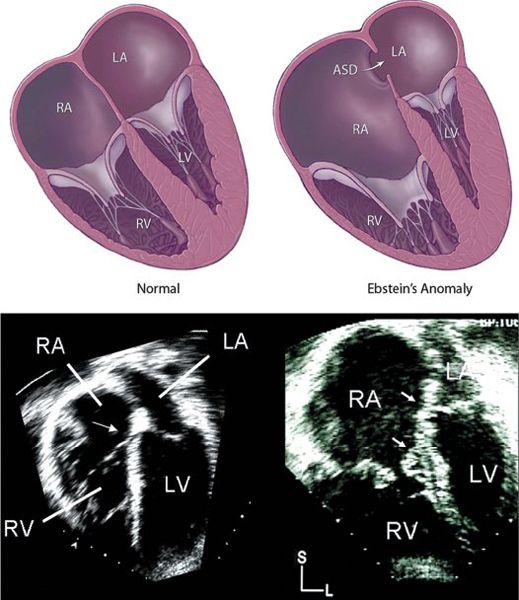
Figure 8.2. Apical four-chamber plane of a normal heart (left) and a heart afflicted with Ebstein’s malformation (right). The hinge point (septal insertion) of the normal STL is positioned slightly more toward the cardiac apex, compared with the septal hinge point of the anterior mitral leaflet (bottom left, arrow). This displacement is exaggerated in hearts with Ebstein’s malformation, as shown by the diagram (top right) and the arrows in the echocardiographic image (bottom right). It should be noted that the valvar leaflets are also abnormal in Ebstein’s malformation. In the case shown (bottom right), the leaflets are thickened and moderately dysplastic. ASD, atrial septal defect; L, left; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; S, superior.
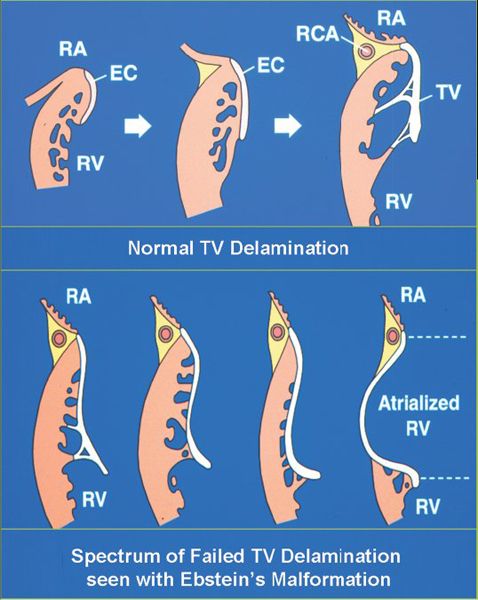
Figure 8.3. Delamination. Normal delamination process that gives rise to the tricuspid valve leaflets (top). During embryonic valve formation, the inner layer of endomyocardium separates (delaminates) from the underlying cardiac muscle and gradually loses its myocardial components. As development progresses (left to right), we begin to recognize the supportive chordal structures and leaflet of the mature valve. When a failure of delamination occurs (bottom), it results in adherence of the “tricuspid valve” tissues to the right ventricular myocardium. This is the hallmark of Ebstein’s malformation. The four diagrams (bottom), progressing from mild to severe (left to right), demonstrate the spectrum of abnormality that can be associated with failed delamination. EC, endomyocardial layer; RA, right atrium; RCA, right coronary artery; RV, right ventricle; TV, tricuspid valve.
In hearts with Ebstein’s malformation, the septal and inferior leaflets are most dramatically involved and show the largest change in the anatomic position of their hinge points. In contrast, the anterior leaflet forms at a different developmental stage. As a result, its junctional hinge usually retains a normal position near the atrioventricular groove and it can become very large and “sail-like.” Mild cases can be encountered in which only the hinge of the septal leaflet is displaced away from the atrioventricular junction. Such cases are found most frequently in the setting of pulmonary atresia with an intact ventricular septum. In the absence of pulmonary atresia, it is unlikely that such minimal changes would produce the typical, if indeed any, symptomatology.
Clinically, it is important to distinguish pathologic displacement of the septal leaflet from the typical valvar offsetting found in the normal heart (see Fig. 8.2). It is in making this distinction that the apical component of the displacement associated with Ebstein’s malformation is most useful. The normal tricuspid septal leaflet hinges at a point on the ventricular septum that is slightly apical to the hinge point of the anterior mitral leaflet, when both are displayed in the apical four-chamber view. Although the two septal hinges are offset, this does not represent displacement of the tricuspid valve. When the septal leaflet is abnormally adherent to the myocardium, as in Ebstein’s malformation, the distance separating the two septal valvar hinge points becomes exaggerated. This exaggerated separation can be quantitated by measuring the linear distance between the two septal hinges in the four-chamber plane. (See discussion of the apical displacement index later in this chapter.)
The abnormalities associated with Ebstein’s malformation also cause the leaflets to adopt a “bileaflet” configuration, with a plane of closure at the junction of the trabecular and the outlet components of the right ventricle (Figs. 8.6, 8.7, and 8.8).
The keys to understanding this malformation and its anatomic consequences are appreciating the developmental etiology of failed delamination and recognizing that the abnormal, displaced location of the valve represents a complex rotational or spiral deformity (Figs. 8.5 and 8.6), rather than the more simplistic linear “apical” displacement that has received attention in the past.
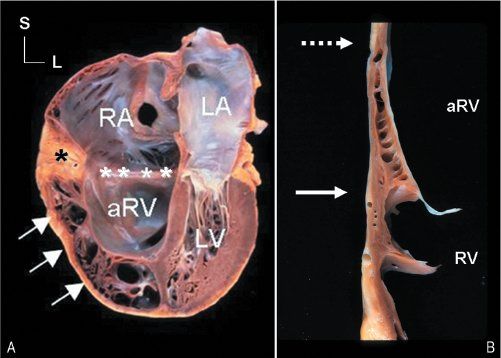
Figure 8.4. Anatomic specimen from a patient with a severe case of Ebstein’s malformation (A) and a segment of right ventricular wall from a patient with Ebstein’s malformation (B). The anatomic atrioventricular junction is marked (asterisks). The failure of the tricuspid valve to delaminate in the specimen not only displaced the valve away from the junction but also produced extensive adherence of the valve tissue to the ventricular myocardium (A, arrows). No mobile segments of valve are appreciated within the right ventricular inlet. The remnant of tricuspid valve tissue (B) is also nearly completely adherent to the underlying myocardium. Only a small tag of tissue shows any separation from the wall (near the solid arrow). The segment of “valve” between the dashed and solid arrows is seen as the dense, white inner lining of the cavity (clearly different from normal endocardial tissue, which can be seen below the level of the solid arrow). The adherence of the tricuspid valve creates a zone of the right ventricular cavity that is “atrialized.” This zone has walls composed of ventricular myocardium, but its cavity is proximal to the tricuspid valvar orifice. aRV, atrialized right ventricle; LA, left atrium; LV, left ventricle; RV, right ventricle; TV, tricuspid valve.
CLINICAL PRESENTATION OF EBSTEIN’S MALFORMATION
Patients with Ebstein’s malformation may present at any age. The most severe cases present prenatally or as newborns. Prenatal diagnosis is dependent on ultrasonic screening examinations. Fetal presentation is accompanied by increased heart size, a significant incidence of fetal hydrops, and, in the most severe cases, pulmonary parenchymal hypoplasia secondary to marked cardiac enlargement. Prenatal arrhythmia is not common. Newborns most often present with cyanosis, while slightly older infants present with a combination of desaturation and symptoms of cardiac failure. Murmurs and arrhythmias are more frequently encountered as presenting complaints in older patients. Although some patients remain asymptomatic, most will have some cardiovascular symptoms. Beyond infancy, the majority will display abnormal fatigability, dyspnea, palpitations, or cyanosis with exertion. Palpitations in a cyanotic child should raise the possibility of Ebstein’s malformation (Table 8.1).
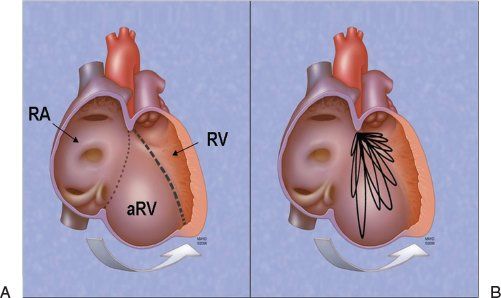
Figure 8.5. Rotation of the functional tricuspid orifice in Ebstein’s anomaly. Rotational shift of the functional tricuspid orifice away from the atrioventricular junction to the plane that divides the trabecular and outlet zones of the ventricle (A, heavy dashed line). The observed planes of the effective valvar orifices of the hearts with Ebstein’s malformation studied by Schreiber and colleagues are shown (B, ovals). The effective displacement of the functional tricuspid valve (heavy dashed line) away from the atrioventricular junction (marked by the dotted line) is rotational, not linear. This rotation is spiral in nature and is directed both toward the apex and, more important, anteriorly toward the outflow tract. aRV, atrialized right ventricle; RA, right atrium; RV, right ventricle.
Echocardiography has significantly influenced the age at which patients with Ebstein’s malformation are diagnosed. In 1979, Giuliani and colleagues found that just under one-third of patients were diagnosed before 4 years of age. Another two-fifths were diagnosed before the age of 19, with the remainder presenting in adulthood, some at 80 years of age. In contrast, in the experience reported by Celermajer et al. in 1994 (see Table 8.1), three-fifths came to clinical attention before the age of 1 year, with half diagnosed prenatally or as newborns. One-tenth presented between 1 and 12 months of age, with only three-tenths presenting as children or adolescents. Despite the increased availability of echocardiographic examination in this more recent cohort, one-tenth remained undiagnosed until adulthood.
ASSOCIATED CARDIOVASCULAR ABNORMALITIES
Nearly all patients with Ebstein’s malformation will have an interatrial communication, with the most frequent being a patent foramen ovale or secundum atrial septal defect. Venosus and partial atrioventricular septal defects do coexist with Ebstein’s malformation but are much less common. A large spectrum of other lesions has been described, including ventricular septal defects, tetralogy of Fallot, aortic coarctation, noncompaction of the left ventricular myocardium, and patency of the ductus arteriosus. The most common nonatrial cardiac abnormality is pulmonary stenosis or atresia, which is found in up to one-third of those presenting in infancy. Obstruction of the right ventricular outflow tract is commonly associated with Ebstein’s malformation when diagnosed in fetal life. In this setting, it may be difficult to distinguish structural from functional pulmonary valve atresia using echocardiography, especially in the presence of severe tricuspid regurgitation. When there is no systolic flow from the right ventricle to the pulmonary artery, the presence of pulmonary regurgitation is the most reliable marker of functional pulmonary atresia (Fig. 8.9). The regurgitant flow can be detected by either color flow or spectral Doppler echocardiography. Doppler detectable pulmonary regurgitation has been reported in more than 80% of series with ductal-dependent neonatal Ebstein’s malformation. In the setting of anatomic pulmonary valve atresia, there is no flow detectable during either phase of the cardiac cycle. In cases of Ebstein’s malformation with either stenosis or atresia, the pulmonary valvar abnormality is probably secondary to the malformation of the tricuspid valve, with hypoplasia of the outflow tract resulting from reduced anterograde flow through the right heart. When severe Ebstein’s malformation is associated with fetal and neonatal distress, or death, both lungs are usually hypoplastic. The markedly enlarged heart compresses the lungs, leading to the pulmonary hypoplasia seen in these cases.
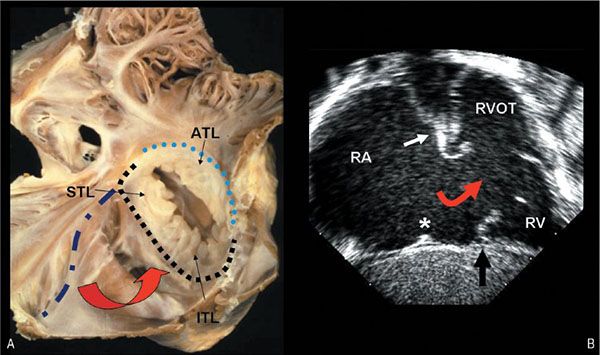
Figure 8.6. Patient with Ebstein’s malformation. A: Anatomic specimen shows the atrial aspect of the deformed valve. It illustrates how the anterior leaflet retains its normal attachment at the atrioventricular junction (light blue dotted line), but the conjoined septal and inferior leaflets have their hinge points attached well away from the atrioventricular junction (compare the dark blue dashed and black dotted lines). The separation of the inferior and septal hinge points from the true annulus is also highlighted by the curved red arrow. The portion of the right ventricle between the darker blue and black lines is said to be “atrialized” and in this case has a very thin wall. Note that the leaflets of the deformed valve will close in “bileaflet” fashion. B: Subcostal, coronal echocardiographic image displays some of the same features. Remnants of the anterior (near the white arrow) and the ITLs (black arrow) are seen. The hinge point of the anterior leaflet (white arrow) retains a normal position, while the insertion of the inferior leaflet is displaced into the ventricular cavity away from the anatomic atrioventricular junction, which is marked (asterisk). Similar to the anatomic image on B, this echocardiogram confirms that the displacement of the tricuspid apparatus associated with Ebstein’s malformation actually rotates the valve anteriorly, as well as apically (curved red arrows). ATL, anterior tricuspid leaflet; ITL, inferior tricuspid leaflet; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; STL, septal tricuspid leaflet.
ECHOCARDIOGRAPHIC RECOGNITION AND EVALUATION OF EBSTEIN’S MALFORMATION
Imaging of the internal cardiac crux, the components of the abnormal tricuspid valve, and the right ventricular myocardium reveals several features that are reliably used to identify patients with Ebstein’s malformation. The single most sensitive and specific diagnostic feature is the displacement of the annular hinge of the septal leaflet. This displacement is most easily appreciated by comparison to the annular hinge of the mitral leaflet as seen in the apical four-chamber view. One must recall that the normal septal tricuspid leaflet inserts at a position that is slightly apical to the insertion of the anterior mitral valve leaflet. In patients with Ebstein’s malformation, this displacement is exaggerated. The distance between the valvar hinge points can easily be measured in systole (Fig. 8.10A). This distance, when divided by the body surface area in square meters, is known as the displacement index. In a heart with evidence of failed delamination, an index value greater than 8 mm/m2 reliably distinguishes those with Ebstein’s malformation from both normals and from patients with other disorders associated with enlargement of the right ventricle. In cases with severely adherent and displaced septal leaflets, the hinge of the septal tricuspid leaflet may not be seen in the four-chamber plane. In these cases, the first septal structure seen on the right ventricular septal surface (apical to the insertion of the anterior mitral leaflet) will be the moderator band (Fig. 8.10B). The apical displacement index is essentially infinite in these hearts, and recognition of the presence of Ebstein’s malformation is straightforward.
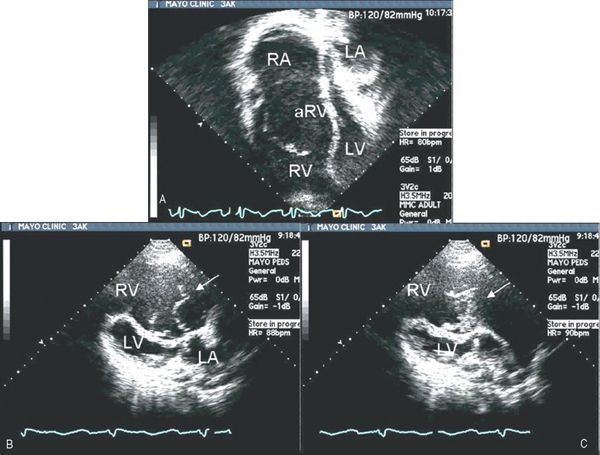
Figure 8.7. Patient with Ebstein’s malformation. A: Four-chamber echocardiographic image. B: Systolic frames in a parasternal long-axis format. The tricuspid valve’s functional orifice does not lie in the “four-chamber” plane but rather has been rotated anteriorly and apically. A cross section of the orifice, as the valve closes, can be appreciated in the long-axis images (arrows). These frames highlight the bileaflet nature of the functional tricuspid valve in Ebstein’s malformation. There is just a single, vertically oriented, line of closure between the functional segments of valve tissue. C: Coaptation gap that allows regurgitant flow. aRV, atrialized right ventricle; LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
Occasionally, it can be difficult to assess the offset between the valvar hinges at the internal crux. In these unusual situations, other echocardiographic features that can assist in making an accurate diagnosis include elongation of the anterior leaflet, tethering of any of the leaflets to the underlying myocardium, shortened chordal supports, attachment of the leading edge of the anterior leaflet to the right ventricular myocardium, displacement of the annular attachment of the anterior leaflet, absence of the septal or inferior leaflets, congenital fenestration of the leaflets, and enlargement of the valvar annulus.
Echocardiography is also used to define associated cardiovascular abnormalities and myocardial function. In addition, preoperative examinations are used to define anatomic features that relate to potential for surgical repair. Over the past decade, circumferential tricuspid valve reconstruction (“cone” repair) has replaced the monoleaflet repair as the preferred surgical approach. Several of the anatomic features that indicated potential success of the monoleaflet repair remain helpful prior to cone reconstructions. The most important feature predictive of a durable repair is the mobility of the anterior leaflet. When 50% or more of the leaflet is freely mobile, there is no major tethering to the myocardium; and due to the leading edge that moves freely within the inlet of the right ventricle, repairs are not only more likely to succeed, but also retain good function longer. These assessments are based primarily on images from the apical four-chamber view (Fig. 8.11), but both subcostal and short-axis views can provide important information as well. Extensive adherence of the anterior leaflet to the ventricular myocardium (see Fig. 8.10B) makes a successful repair less likely. A single central jet of regurgitation (Fig. 8.12) is more easily eliminated than are multiple regurgitant orifices (Fig. 8.13). Even when there is a significant amount of leaflet tissue present, direct muscular insertions from the ventricular free wall into the leading edge of the anterior leaflet can make cone repairs more difficult and often lead to failure of the monoleaflet approach (Fig. 8.14). Figures 8.15 and 8.16 demonstrate the results of successful monoleaflet repairs.
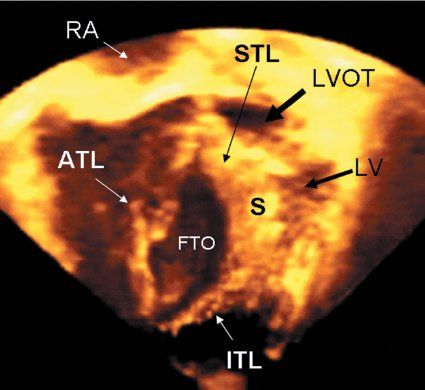
Figure 8.8. Three-dimensional echocardiographic image, from a transthoracic examination, has been cropped to show a view analogous to an apical long-axis image.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


