Fig. 13.1
Incidence of in-stent restenosis after carotid artery stenting (CAS). (a) Kaplan-Meier cumulative event rates for clinically significant ISR ≥ 80% after CAS. (b) Kaplan-Meier cumulative event rates for ISR ≥ 60% after CAS. (c) Kaplan-Meier cumulative event rates for ISR ≥ 40% after CAS. Number of patients at the beginning of each time interval and standard error are indicated below the X-axis of each graph. N number at risk, SE standard error (Adapted from Lal et al. [10]. With permission from Elsevier)
Natural History
Carotid Endarterectomy
The clinical significance of CR after CEA is still debated. Symptomatic CR clearly benefits from repeat revascularization. However, the risk of stroke or progression to total occlusion [2, 7] is known to be low in patients with asymptomatic CR. Therefore, some clinicians have proposed careful surveillance alone for asymptomatic patients [12]. Conversely, most surgeons elect to reintervene on high-grade (≥70%) asymptomatic lesions. The rationale for such an approach is that it is extremely difficult to predict which preocclusive lesions will remain asymptomatic [13, 14]. We also subscribe to this view with respect to restenosis after CEA [15].
Carotid Artery Stenting
There is limited information on what factors constitute risks for in-stent restenosis (ISR) after CAS. We therefore developed a novel protocol to assess and classify the morphologic patterns of ISR after CAS [16] into types I (focal ≤ 10 mm, end-stent lesions), II (focal ≤ 10 mm, intrastent), III (diffuse > 10 mm, intrastent), IV (diffuse > 10 mm, proliferative, extending outside the stent), and V (total occlusion) (Fig. 13.2). Eighty-five ISR lesions developed after 255 CAS procedures. Their percent distributions were 40, 25.9, 12.9, 20, and 1.2 (types I through V, respectively). The accuracy of our US classification was confirmed by angiography (r 2 = 0.82). Thirteen lesions were ≥80% diameter reducing and required endovascular reintervention. On multivariate analysis, only the type of ISR (odds ratio, 5.1) and a history of diabetes (OR, 9.7) were independent predictors of high-grade recurrent ISR and reintervention [16]. Furthermore, there was a significant increase in reintervention in association with increasing levels of ISR classification (0%, 0%, 27.3%, and 58.8% for types I–IV, respectively; χ 2 trend = 29.4, p = 0.001). Follow-up duplex US evaluations after CAS must therefore include an assessment of the morphologic pattern of ISR. While additional prospective data is required to confirm these findings, it is likely that patients with type IV lesions will benefit from a more intensive duplex surveillance program (perhaps every 6 months for life).
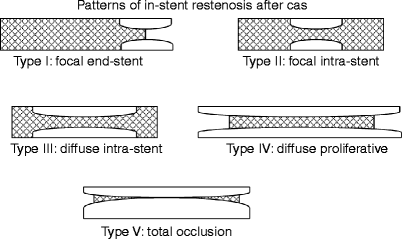

Fig. 13.2
Classification of the morphologic patterns of carotid in-stent restenosis. The classification is based on the length and geographic location of the neointimal hyperplastic lesion with respect to the stent. The shaded area represents the stent (Adapted from Lal et al. [16]. With permission from Elsevier)
DUS Velocity Criteria
Carotid Endarterectomy
Duplex ultrasonography (DUS) is the standard technique to follow carotid stenosis patients treated with CEA or medical therapy alone. Duplex ultrasonography offers several advantages in the follow-up of patients treated with carotid revascularization. It is noninvasive, safe, free of complications, readily available in vascular laboratories around the country, and is associated with a large experience with primary and recurrent carotid stenosis of the native carotid artery. The utility of DUS scanning in the detection of native carotid artery disease is well documented and has led to the use of peak-systolic velocities (PSV), end-diastolic velocities (EDV) and PSV/EDV ratio, or internal carotid/common carotid artery PSV ratios (ICA/CCA ratios), alone or in combination, to define normal and increasingly stenosed ICAs. Ultrasound velocities correlate with angiographic percent stenosis in the native carotid artery, and the appropriate threshold velocities signifying different degrees of stenoses have been intensively analyzed and identified [17–19].
Carotid Artery Stenting
Duplex ultrasound velocity criteria had not been well established for patients undergoing CAS until very recently. Two studies initially reported altered blood flow velocities after carotid stent placement. The authors proposed that these variations in velocity measurements adversely affected the accuracy of duplex ultrasonography (DUS) in CAS patients. They concluded that DUS velocity measurements as an index of stenosis were not reliable after carotid stent placement [20, 21].
Carotid Stenting Alters Compliance
In 2004, we reported that the placement of a stent altered the biomechanical properties of the carotid territory such that compliance was reduced [22] (Fig. 13.3). Compliance is a material property measured by the relationship between strain (fractional deformation of wall) and stress (force per unit area of wall). In an artery, compliance is described by the change in volume of a segment of artery in relation to pulsatile change in blood pressure. Assuming a cylindrical conformation for the arterial segment, the volume may be calculated as πr 2 l (r = radius; l = length). Since length is usually constant, the relative diameter change per unit pressure has been used as an index of changes in compliance. We also found that the enhanced stiffness of the stent-arterial wall complex rendered the flow-pressure relationship of the carotid artery closer to that observed in a rigid tube [23] so that the energy normally applied to dilate the artery resulted in an increased velocity.
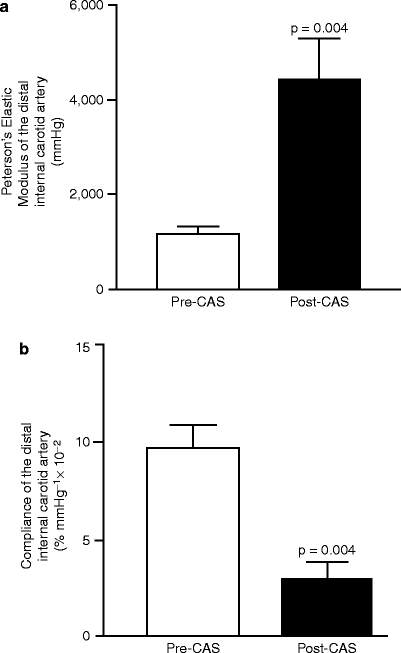

Fig. 13.3
Carotid artery stenting alters the biomechanical properties of the stent-arterial complex. Measurement of elastic modulus (a) and compliance (b) of the native internal carotid artery versus stented internal carotid artery (Adapted from Lal et al. [22]. With permission from Elsevier)
Stenting Increases Velocity Measurements
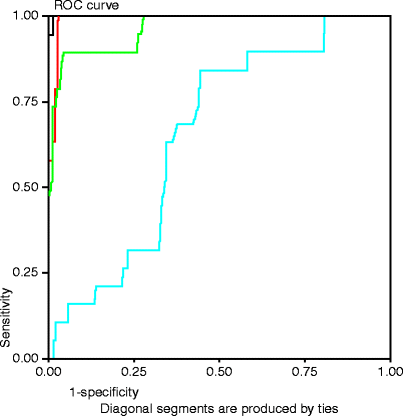
In this first report, we compared post-CAS ultrasound velocities with angiographically measured residual in-stent stenosis after 90 CAS procedures. Mean angiographic residual stenosis after CAS was 5.4%, while corresponding PSV on DUS was 120.4 cm/s; EDV, 41.4 cm/s; PSV/EDV ratio, 3.3; and ICA/CCA ratio, 1.6. Receiver operating characteristic (ROC) analysis demonstrated that a combined threshold PSV ≥ 150 cm/s and ICA/CCA ratio ≥2.16 were optimal for detecting residual stenosis ≥20%. Based on these observations, we concluded that DUS was useful and accurate in identifying stenotic lesions in stented carotid arteries. However, revised velocity criteria would need to be developed to identify higher grades of ISR in this situation.
Velocity Thresholds for Restenosis
These observations were followed by at least four studies that addressed this hypothesis. Peterson et al. [24] analyzed DUS velocity and angiographic measurements of stenosis in three patients with high-grade ISR and proposed new criteria defining ISR ≥ 70% (PSV > 170, EDV > 120, and velocity increase >50% over baseline). Stanziale et al. [25] analyzed velocity/angiography observations obtained primarily from procedural angiography and selected angiography performed in patients with suspected high-grade ISR during follow-up. They proposed new criteria defining ISR ≥ 70% (PSV ≥350 and ICA/CCA ratio ≥ 4.75) and ISR ≥ 50% (PSV > 225 and ICA/CCA ratio ≥ 2.5). Chi et al. [26] analyzed 13 pairs of DUS and angiogram observations in CAS patients with suspected high-grade ISR. They offered alternate criteria to define ISR ≥ 70% (PSV ≥ 450, or ICA/CCA ratio ≥ 4.3) and ISR ≥ 50% (PSV ≥ 240 or ICA/CCA ratio ≥ 2.45). Chahwan et al. [27] analyzed six pairs of observations from patients with high-grade ISR on follow-up along with procedural angiograms (n = 71). They concluded that a normal DUS after CAS is reliable in identifying a normal artery, but larger studies would be required to determine appropriate threshold criteria. These studies indicated that higher grades of restenosis were also overestimated in the stented artery when velocity criteria for native arteries were utilized. However, procedural risks precluded routine angiographic follow-up, and comparisons could not be performed across the full spectrum of degrees of restenosis. This explained the wide variation in proposed threshold velocity criteria.
Our group [28] later compared DUS velocity measurements with luminal stenosis measured by either angiography or CT angiography (CTA) during follow-up of all our CAS patients (n = 310 observations). Patients were followed with annual DUS and CTA at their most recent follow-up visit. Patients with suspected high-grade ISR on US underwent diagnostic carotid angiography. The DUS protocol included peak-systolic (PSV) and end-diastolic velocity (EDV) measurements in the native common carotid artery (CCA), proximal stent, mid stent, distal stent, and distal internal carotid artery (ICA). The accuracy of CTA versus CA was confirmed (r 2 = 0.88). Post-CAS PSV (r 2 = 0.85) and ICA/CCA ratios (r 2 = 0.76) correlated most with the degree of restenosis. Receiver operator characteristic (ROC) analysis demonstrated the following optimal threshold criteria: residual stenosis ≥20% (PSV ≥ 150 cm/s and ICA/CCA ratio ≥ 2.15), ISR ≥ 50% (PSV ≥ 220 cm/s and ICA/CCA ratio ≥ 2.7), and ISR ≥ 80% (PSV ≥ 340 cm/s and ICA/CCA ratio ≥ 4.15) (Fig. 13.4). We therefore proposed revised velocity criteria [28] for identifying restenosis in stented carotid arteries (Table 13.1). While our results can be used as guidelines, individual laboratories must develop threshold criteria that are accurate for their own environment. These proposed criteria can form the basis for additional prospective validation studies.