Fig. 49.1
Lateral view demonstrating the typical ostial location of most atherosclerotic lesions in the main mesenteric vessels. There is a severe stenosis of both the celiac (larger arrow) and superior mesenteric arteries (smaller arrow) near their origins from the aorta
Mesenteric Duplex Scanning: Technique
As with all deep abdominal duplex scans, intestinal gas will compromise the technical success of mesenteric examination. An overnight fast is adequate preparation for most elective cases, but simethicone-containing compounds may be useful in cases where bowel gas remains a problem. In patients with a more acute problem, the ileus produced by any intra-abdominal inflammatory processes (e.g., acute mesenteric ischemia, cholecystitis, pancreatitis, diverticulitis) greatly limits the usefulness of mesenteric scanning. When a technically adequate examination can be performed it may be useful in directing further diagnostic evaluation, but in emergent cases where mesenteric ischemia is suspected, arteriography should be performed without delay.
Mesenteric duplex scanning is performed with the patient supine and the head slightly elevated. Low frequency, dedicated abdominal probes are used for mesenteric, renal, hepato-portal, and vena caval scanning. An anterior-posterior midline approach is used to obtain a sagittal scan of the aorta. The origins of the CA and SMA are usually visualized as they course ventrally from the aorta (Fig. 49.2) above the level where the left renal vein crosses the aorta. Most atherosclerotic occlusive lesions in the CA and SMA are at or near the origins of the vessels from the aorta, so insonation of the first few centimeters of each vessel is usually adequate for diagnosis. The inferior mesenteric artery (IMA) originates from the left side of the infrarenal aorta a few centimeters above the aortic bifurcation.
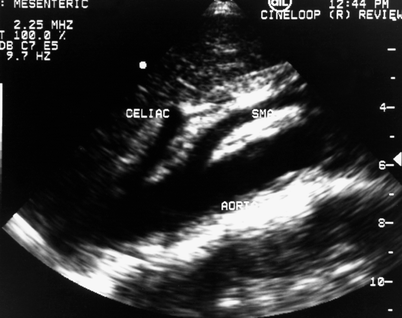

Fig. 49.2
Sagittal scan of the aorta in a normal patient is similar to the lateral aortogram and demonstrates the origins and proximal portions of the celiac trunk and the superior mesenteric artery. Pulsed Doppler sampling is performed in the proximal portions of these vessels where most occlusive disease occurs. SMA superior mesenteric artery
Pulsed Doppler examination is performed using a 1.5–2.0-mm-sample volume. Peak systolic velocity (PSV), end diastolic velocity (EDV), waveform configuration, and direction of flow are recorded. Doppler angles of insonation = 60° must be employed when sweeping through the vessels. Rizzo et al. [7] observed that pulsed-Doppler arterial flow velocities from the mesenteric vessels should be measured at angles of insonation less than 60° or falsely elevated peak systolic velocities will be recorded even in normal vessels. The anatomy of the vessels often produces sudden changes in vessel direction and it is incumbent upon the technologists to be as certain as possible about the location and angle of the sample.
Accurate examination of the celiac trunk may be challenging. The celiac trunk is rarely longer than 1–2 cm and the anatomy of its branches (common hepatic, splenic, and left gastric) may be extremely variable. The left gastric artery is rarely identified by duplex scan and routine examination includes the celiac, hepatic, and splenic arteries. While flow in the origin of the CA roughly parallels that in the SMA ventral from the aorta, the branching of the celiac trunk results in rapid changes in the direction of arterial flow at almost 90° to the right (hepatic) and left (splenic) of the main celiac trunk. These anatomic relationships can be appreciated with a transverse B-mode scan of the CA, which has been termed the “rabbit-ear” or “seagull” appearance (Fig. 49.3).
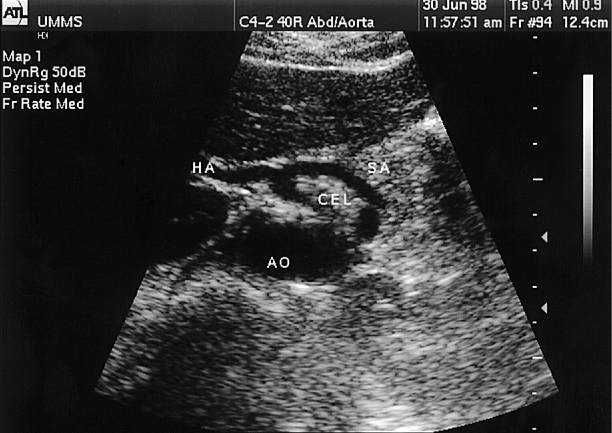

Fig. 49.3
Doppler spectral analysis in the celiac trunk (CEL) may be challenging due to changes in the angle of insonation produced by its branching into the splenic artery (SA) and the common hepatic artery (HA). These sudden changes in the direction of flow in the celiac branches can produce falsely elevated flow velocities unless the angle of insonation is carefully controlled at = 60°. Ao aorta
Identification and pulsed-Doppler spectral analysis is easier in the SMA than in the celiac trunk. There are generally no major branches of the SMA visualized on routine examination. However, a “replaced right hepatic artery” may originate from the SMA in up to 20% of normal cases. Some patients may even have a common trunk origin of both the CA and SMA. The variable collateral patterns that are present in cases of occlusion of a single main mesenteric artery may be even more confusing. Large gastroduodenal or pancreaticoduodenal branches may serve as collateral communication between the CA and SMA and these may be difficult to interpret by an inexperienced examiner.
The IMA has not been routinely studied but may be identified by scanning down the infrarenal aorta toward the bifurcation where it is generally the only vessel originating from the left side of the aorta. Isolated stenosis or occlusion of the IMA is rare but it may play a major role in the visceral collateral circulation. The presence of a markedly enlarged and thereby, easily identifiable IMA may suggest significant occlusive disease of the SMA with the IMA serving as the collateral (Fig. 49.4). However, many patients with mesenteric arterial disease have associated aortoiliac occlusions. Here, the IMA may be occluded, but large lumbar collaterals may be mistaken for the IMA on duplex scanning. Another potential source of error in attempt to scan the IMA is the presence of an accessory lower pole left renal artery, which would also originate from the left side of the infrarenal aorta. Overall, in most cases it is sufficient to focus the examination on occlusive lesions at the origins of the CA and SMA, since this is the area of involvement in most clinically relevant disease.
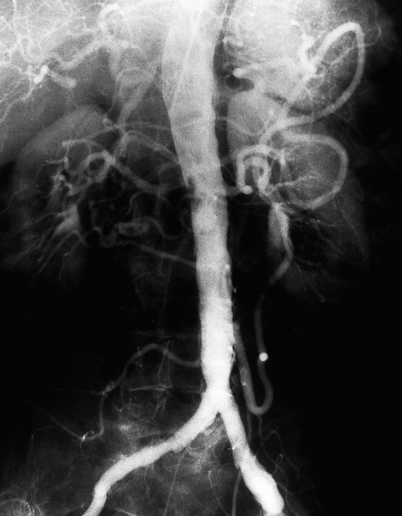

Fig. 49.4
Aortogram demonstrating a dramatically enlarged inferior mesenteric artery (IMA) originating from the left side of the infrarenal aorta several centimeters above the aortic bifurcation. Retrograde flow through the “meandering mesenteric” collateral is demonstrated in this patient with an SMA occlusion
Color Doppler scanning allows more rapid identification of the origins of the CA and SMA from the abdominal aorta and will reduce the time required for an examination. Color-flow scanning may help visually identify focal areas of flow disturbance that require further interrogation, or help one detect the absence of flow in one or both of the major mesenteric vessels suggesting occlusion. It is important to remember, however, that color assignment is based almost entirely upon direction of flow. The anatomic variations discussed above make it evident that beyond the origins of these vessels, sudden changes in flow direction can produce confusing color-flow patterns even in normal subjects.
Reliable, broadly accepted diagnostic Doppler frequency, or flow velocity parameters have not been developed for the mesenteric circulation as they have been in most other systems (carotid, renal, bypass grafts, etc.). Most reports of visceral arterial duplex scanning emphasize the determination of PSV, EDV, and the presence or absence of early diastolic reversal of flow. Similar to carotid scanning, significant stenoses are most often identified by a focal increase in systolic and diastolic velocities, and occlusions are identified by the absence of flow, or flow reversal. Unlike renal artery duplex scanning, no improvement in diagnostic accuracy has been observed by normalizing visceral arterial velocity measurements to those in the aorta through the calculation of flow velocity ratios [8, 9]. Some investigators have reported quantitative blood flow (ml/min) measurements using Doppler derived velocity information in either the portal venous system [10, 11], or in the mesenteric arterial circulation [12–15]. Although there is considerable research interest in volumetric flow data, this estimation is subject to significant error [16–18], and most diagnostic laboratories do not perform such measurements. The presence of elevated PSV and localized turbulence appears to correlate better with angiographically demonstrated arterial lesions in both the peripheral and the central circulation.
Normal Findings
Mesenteric arterial velocity waveforms have certain specific characteristics. At rest, blood flow in a normal SMA has a higher resistance Doppler velocity pattern with early diastolic flow reversal in most cases, and late diastolic forward flow (triphasic waveform; Fig. 49.5). Blood flow in the CA as in the renal arteries, has a low resistance Doppler velocity pattern with continuous forward flow during the entire cardiac cycle (Fig. 49.6). Spectral waveforms recorded from normal IMA are similar to those from the SMA with a higher resistance pattern with early diastolic flow reversal.
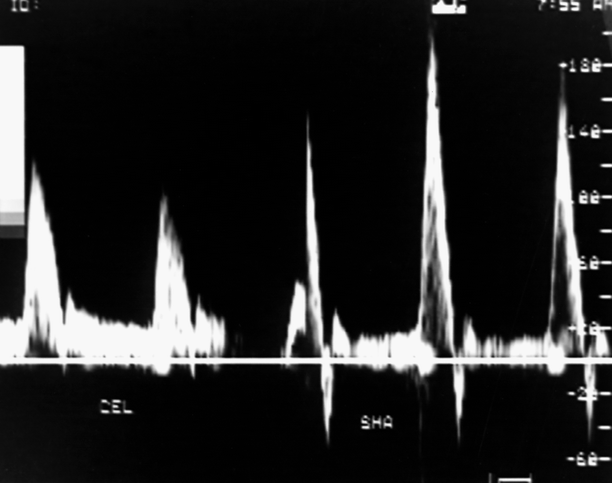
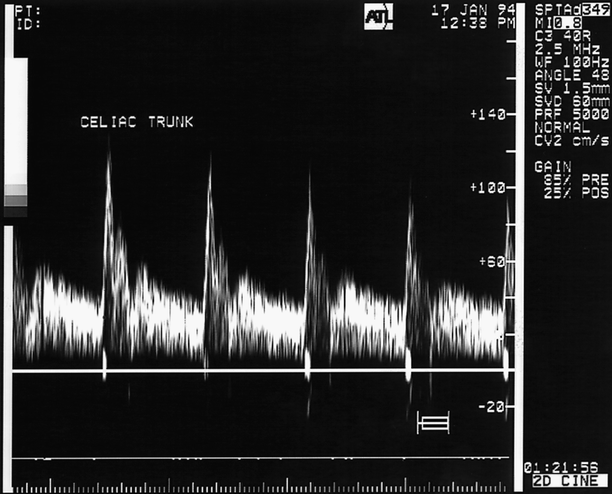

Fig. 49.5
The normal fasting SMA waveform (right spectra) is recognizably different from the celiac artery (CEL, left). The triphasic pattern of the normal SMA is reminiscent of that found in higher resistance peripheral arteries

Fig. 49.6
Velocity spectrum from a normal celiac artery demonstrating continuous forward flow throughout systole and diastole
Clinical Applications
Physiologic Measurements
Duplex scanning of the mesenteric vessels in normal individuals has been used successfully to characterize the physiological changes that occur in the visceral circulation after eating. The most reproducible changes occur in the SMA, where significant increases in PSV are seen up to an hour after eating [19]. Also, the SMA flow waveform shifts to a low resistance pattern characterized by forward flow throughout the cardiac cycle with loss of the early diastolic flow reversal. The composition of the meal, including volume, energy content, and the nutritional composition, may influence the observed changes in mesenteric flow velocities after a meal [1, 4, 15, 20]. Fat and carbohydrates appear to be the nutritional components of the meal that produce the most significant postprandial increases in measured PSV [15].
Duplex scanning has also been used to demonstrate the effects of drugs on intestinal blood flow. Several investigators have demonstrated changes in mesenteric arterial flow following the infusion of splanchnic vasodilators such as glucagon and secretin, and vasoconstrictors such as vasopressin [19, 21]. Lilly et al. [19] observed that the changes in superior mesenteric arterial flow following glucagon infusion closely paralleled those observed after a meal.
Visceral Aneurysms
Aneurysms of the mesenteric vessels are extremely rare and the clinical usefulness of duplex scanning for the detection of mesenteric aneurysms remains anecdotal. Ultrasonographic diagnosis of aneurysms of the superior mesenteric [22–24], hepatic [25, 26], splenic [27, 28], gastroduodenal [29], middle colic [30], and pancreaticoduodenal [31] arteries has been reported, but in these cases scanning was often performed due to uncertain gastrointestinal or abdominal complaints. However, duplex scanning may be useful in such cases to select patients for more thorough, focused vascular exam with CT, MRI, or arteriography. Color-flow duplex ultrasonography may be useful for differentiating saccular false aneurysms of the superior mesenteric and splenic artery from other more benign, nonvascular fluid collections in the setting of pancreatitis or other retroperitoneal inflammatory conditions. However, as noted above, an associated ileus with excessive bowel gas may preclude a diagnostic study.
Celiac Artery Compression Syndrome (Median Arcuate Ligament Syndrome)
A focal increase in flow velocities identified at the origin of the CA may be due to extrinsic compression of the CA by the median arcuate ligament of the diaphragm, particularly when this finding is detected in a younger individual. Celiac artery compression syndrome has been reported to produce gastrointestinal symptoms in some patients, but most clinicians regard this as a benign condition with little or no risk of intestinal infarction. In fact, duplex scanning of these lesions is more likely to be performed to evaluate the finding of the celiac stenosis on an arteriogram performed for other reasons (Fig. 49.7). This “lesion” is usually associated with a normal arterial wall and normal aorta with no evidence of atherosclerotic plaque. Scanning during deep inspiration with breath holding produces a relaxation of the diaphragmatic crus and often results in a return of normal celiac velocities, which confirms the diagnosis.
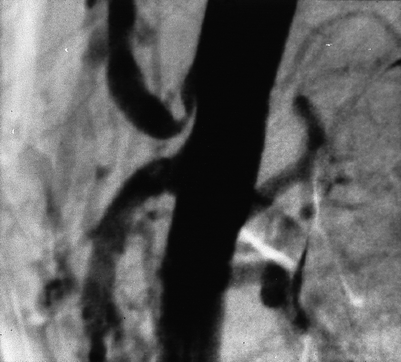

Fig. 49.7
Characteristic arteriographic appearance of proximal celiac artery stenosis due to compression by the median arcuate ligament of the diaphragm. Note there is no evidence of atherosclerosis in the aorta or the SMA. Deep inspiration in this case produced a normalization of both duplex scan findings and the arteriogram
Visceral Ischemic Syndromes
The use of duplex ultrasonography as a screening test to detect major mesenteric arterial occlusive disease in patients suspected of having chronic intestinal ischemia has attracted interest among clinicians, since the diagnosis of mesenteric occlusive disease in the past required arteriography. Jäger et al. [32] first reported abnormal pulsed Doppler waveforms with increased PSVs and marked spectral broadening and in both the CA and SMA of a patient with severe atherosclerotic stenosis in both vessels and symptoms of chronic visceral ischemia. Other authors [33, 34] have used similar criteria of distorted Doppler flow patterns or absence of detectable flow to document high-grade stenosis or occlusion of the intestinal arteries. Moneta et al. [35] reported ultrasound visualization of an enlarged IMA in several patients with high-grade stenosis or occlusion of the celiac and superior mesenteric vessels where that vessel had become the major collateral supply to the bowel.
Standardized velocity criteria for duplex scan diagnosis of hemodynamically significant lesions of the mesenteric arteries (similar to those used in the diagnosis of extracranial carotid artery disease) have not been thoroughly refined. Considering the low prevalence of mesenteric disease compared to carotid disease and the small number of arteriograms available for comparison, it would appear unlikely that such discrete diagnostic criteria would be forthcoming soon. Nevertheless, several studies can provide general ranges for diagnosis which will be clinically useful for the practitioner. Moneta et al. [9] compared the results of mesenteric duplex scanning to arteriography in 34 patients with known atherosclerosis. This group included patients with suspected visceral ischemia as well as others requiring routine arteriography for lower extremity ischemic symptoms. An analysis of their accumulated data revealed that PSVs above 275 cm/s in the SMA (normal = 125–163 cm/s) could predict a severe SMA stenosis (>70%) with a sensitivity and specificity of 89% and 92%, respectively. A similar diagnostic accuracy was observed by these investigators when PSVs exceeded 200 cm/s in the CA. Total occlusions of the CA and SMA were also accurately diagnosed by duplex scan in this report. This initial retrospective study failed to demonstrate the usefulness of a “mesenteric:aortic” systolic velocity ratio to predict the presence of a severe stenosis in the CA or SMA as has been observed in duplex scanning of the renal arteries. In a subsequent report [36], these investigators prospectively evaluated these diagnostic criteria in 100 patients having arteriograms and demonstrated that mesenteric duplex scanning was indeed sufficiently accurate to be clinically useful as a screening examination in cases with suspected CA or SMA occlusive disease (Fig. 49.8).
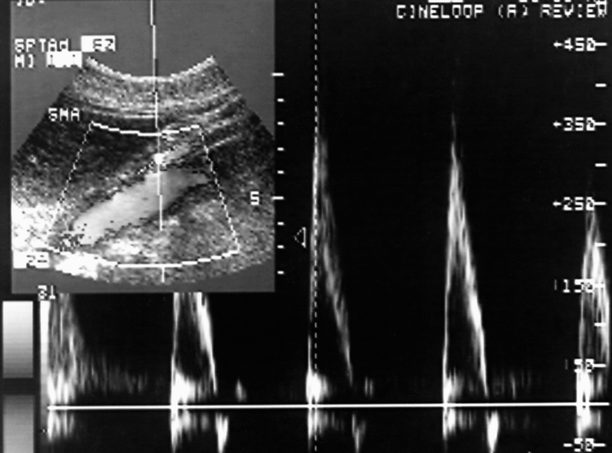

Fig. 49.8
Duplex scan of the proximal SMA reveals a markedly elevated peak systolic velocity >350 cm/s. This suggests the presence of a >70% SMA stenosis
The usefulness of mesenteric duplex scanning for the diagnosis of significant CA and SMA lesions was confirmed in the report of Bowersox et al. [37] that compared mesenteric duplex scanning with arteriograms in 25 patients, most of whom were suspected of having visceral ischemia. These investigators observed that a >50% stenosis of the SMA could be best predicted by duplex scan measurement of a fasting PSV > 300 cm/s or an EDV of >45 cm/s. These authors were unable to establish reliable duplex scan velocity criteria for CA stenosis. They observed that the anatomy of the CA compromised precise insonation as noted previously in this chapter. Considering the small sample size and the fact that most patients were symptomatic, it is possible that anatomic and collateral variants would account for the observed compromise. Further emphasizing the difficulties encountered in an attempt to define precise duplex ultrasound diagnostic criteria for mesenteric occlusive lesions, Healy et al. [38] were unable to identify any definitive velocity criteria for detection of CA or SMA stenoses.
These observations generally serve to reinforce the continued need for prospective studies with arteriographic correlation. However, as noted above, cases available for study are seen infrequently. Nevertheless, the general principles of duplex scan detection of a “critical” stenosis or occlusion in any arterial system apply in the mesenteric vessels: (1) a focal marked elevation in PSV, particularly associated with elevated EDV, (2) post-stenotic turbulence with reduced flow velocities beyond the stenosis, and (3) absence of flow in an anatomically well defined arterial segment, particularly with flow reversal beyond the lesion (suggestive of occlusion). The key to success in the mesenteric circulation relies not so much upon precise velocity criteria, but in accurate anatomic identification of the vessels and control of the technologic variables for Doppler examination.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


