Graft type
Number
Peak systolic velocity (cm/s)(mean ± SD)
In situ saphenous vein
Femoropopliteal
65
76 ± 12
Femorotibial
95
72 ± 16
Femoral-pedal
25
52 ± 12
Reversed saphenous vein
Femoropopliteal
20
80 ± 16
Femorotibial
12
69 ± 14
Cephalic/basilic arm vein
Femoropopliteal/tibial
68
63 ± 12
PTFE, 6 mm diameter
Femoropopliteal
20
80 ± 16
Femorotibial
12
69 ± 14
PTFE, 5 mm diameter
Femorotibial
5
77 ± 11
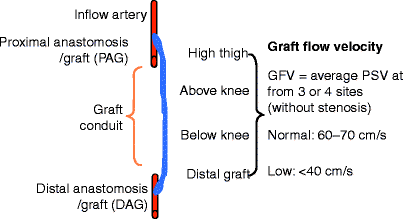
Fig. 24.1
Schematic depicting the recording sites used for the calculation of mean graft flow velocity
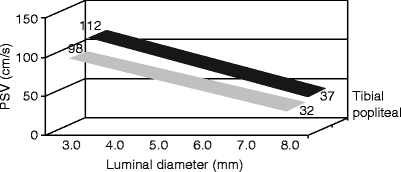
Fig. 24.2
Duplex-ultrasound-measured peak systolic flow velocity (PSV) for tibial and popliteal grafts showing relationship with lumen diameter
A graft flow pattern of low outflow resistance is prognostic of successful bypass grafting. A bypass graft performed for critical limb ischemia should demonstrate this “hyperemic” flow pattern in the distal graft segment and runoff artery, while a bypass performed for claudication may demonstrate triphasic flow immediately. The hyperemia of limb revascularization abates within weeks with the distal graft spectral waveform developing to a triphasic flow pattern if ABI has normalized (Fig. 24.2). Bypasses to the peroneal artery often retain monophasic flow due to collateral flow to the leg and foot. Failure of the graft flow to convert from a biphasic to triphasic spectral waveform has been associated with reduced graft patency. Taylor et al. reported that 54 (86%) of 63 grafts with persistent monophasic waveforms beyond 3 months were frequently found to have developed a graft stenosis or subsequently occluded [5]. In patients with dialysis-dependent end-stage renal disease and severely calcified tibial arteries, a low-flow (PSV in the range of 30–45 cm/s) bypass with minimal or no diastolic flow can occur due to diseased runoff, its associated high outflow resistance, and reduced artery wall compliance.
The identification of changes in graft PSV is important diagnostic criteria for detection of the “failing” bypass graft. Development of a low (<40–45 cm/s) PSV or a decrease in PSV of >30 cm/s in mean graft velocity compared to a prior study indicates a significant change in bypass flow, and a careful search for a graft stenosis should be initiated (Fig. 24.3). The duplex features of a >70%, pressure-reducing stenosis included lumen reduction with color Doppler aliasing, PSV increase to >300 cm/s with spectral broadening, and a PSV ratio (at stenosis compared to proximal to stenosis) >3.5 (Figs. 24.4and 24.5). The increase in diastole flow velocity is due to flow limitation during systole requiring flow throughout the pulse cycle to supply adequate volume flow to the distal limb. If duplex scanning of the bypass does not identify a stenosis, graft imaging by contrast arteriography or computed tomography (CT) angiography is recommended. Elevated PSV (>150 cm/s) in the graft conduit or anastomosis is abnormal and may be caused by small graft caliber or a stenosis. Graft PSV in the range of 120–160 cm/s can be measured along the entire course of small (3 mm diameter or less) vein conduits. More frequent (every 4–6 weeks) surveillance of these “high”-velocity grafts is recommended because of their propensity to develop long segment strictures. When a progressive increase in PSV to >300 cm/s is identified, replacement of the stricture with an interposition vein bypass should be performed as balloon angioplasty has been found to be a less durable repair.
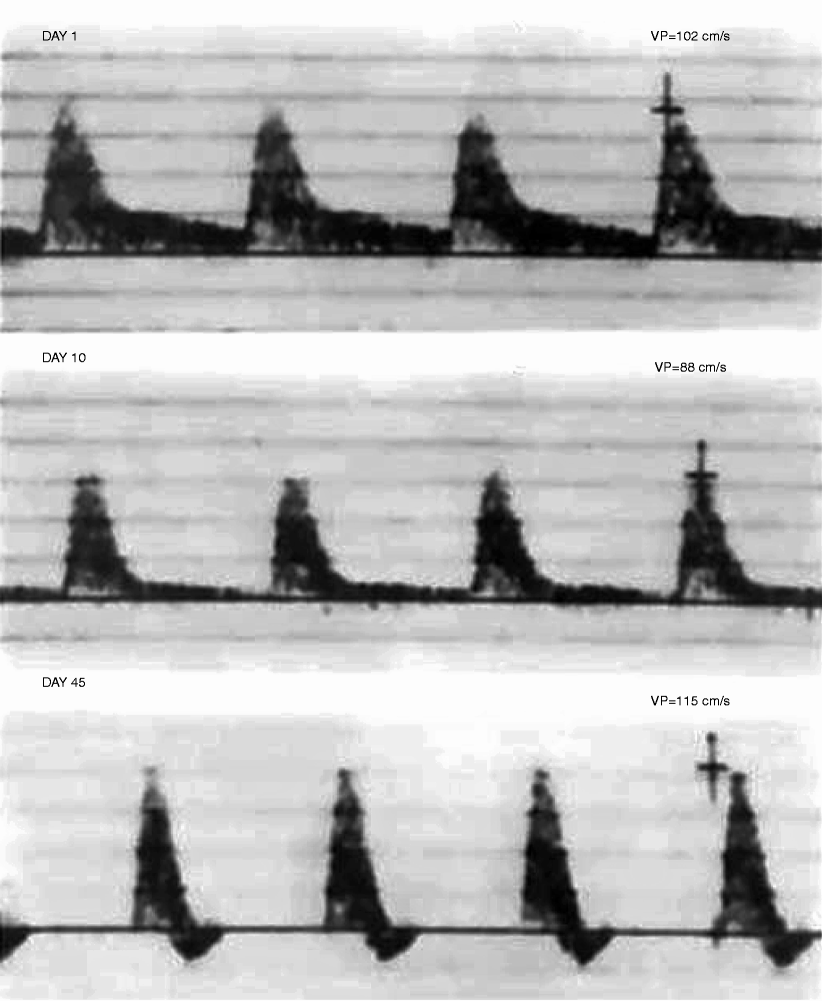
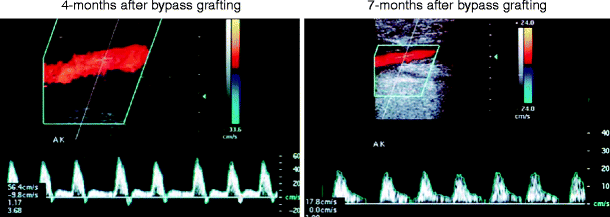
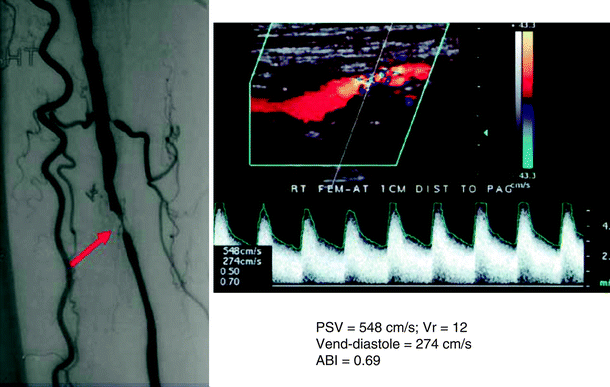

Fig. 24.3
Velocity spectra recorded from the distal segment of a femoral-peroneal in situ saphenous vein bypass at 1, 10, and 45 days. A low-resistance spectral waveform (flow throughout the pulse cycle) is normal and expected after bypass grafting for critical ischemia. Flow resistance increased with time as evidenced by the decrease in diastolic flow. No significant change in peak systolic velocity (Vp) occurred, and the development of a triphasic spectral waveform at day 45 indicates normal bypass graft hemodynamics and distal limb perfusion (ABI > 0.9)

Fig. 24.4
Duplex-recorded velocity spectra recorded from the above-knee segment of a femoropopliteal vein bypass, demonstrating change in waveform configuration (triphasic to biphasic) and reduction in peak systolic velocity (PSV) from 56 to 18 cm/s caused by the development of a distal anastomotic graft stenosis

Fig. 24.5
Angiogram and duplex ultrasound scan of a >75% diameter reducing stenosis of the proximal segment of a superficial femoral artery to anterior tibial artery vein bypass. Peak systolic velocity (PSV) is 548 cm/s, velocity ratio is 12, end-diastolic velocity is 274 cm/s, and ABIis 0.69 decreased from the initial postoperative value of 0.95 Critical stenosis of femoral artery (arrow)
The development of graft stenosis and impending graft occlusion can be recognized from the graft spectral waveform. A low-velocity, biphasic waveform is the most common configuration associated with an acquired graft stenosis (Fig. 24.3). An abnormal graft waveform of this type is recorded from 50% of grafts with stenosis; the ABI had decreased to 0.4–0.7, and mean graft velocity (MGV) has decreased by 20–30 cm/s. The reduction in waveform pulsatility and return of diastolic flow indicate arteriolar dilation in response to a pressure-reducing lesion. Other types of abnormal graft waveforms include a monophasic waveform with low (<45 cm/s) PSV seen in one-third of graft stenosis with an ABI in the range of 0.7–0.9, and a staccato graft waveform seen in approximately 6% of abnormal grafts and always associated with a high-grade outflow stenosis. This staccato flow pattern indicates a to-and-fro blood flow within a compliant venous conduit, extremely low graft flow, and impending graft thrombosis. Grafts with this flow feature are difficult to evaluate using contrast arteriography, and the condition has been referred to as a graft “pseudoocclusion.” The combination of graft PSV measurements, color duplex imaging for anatomic lesions, and ABIs analyzed sequentially during the postoperative period provides a comprehensive characterization of graft and limb hemodynamics. The objective data provided by duplex testing allows detection of graft stenosis, the progression of lesions, and identification of grafts at increased risk for thrombosis.
Intraoperative Duplex Scanning
Assessment of infrainguinal bypass grafts at operation for technical adequacy can be performed using continuous-wave (CW) Doppler flow analysis, arteriography, angioscopy, and CW Doppler after flow is restored, or color duplex ultrasonography [22–24]. Color duplex ultrasound is the preferred method because it provides both anatomic and hemodynamic assessment of graft function and limb perfusion. When used at operation, approximately 15% of procedures have an unrecognized abnormality with velocity spectra of a stenosis identified. Repair of these problems and documentation of normal graft hemodynamics are associated with a low (<1%) incidence of graft thrombosis. In a series of 626 consecutive infrainguinal vein bypasses, duplex scanning prompted revision of 104 lesions in 96 bypass grafts, which included 82 vein/anastomotic stenoses, 17 vein segments with platelet thrombus, and 5 low-flow grafts. The revision rate was highest (p < 0.01) for alternative vein bypass grafts (27%) compared with the other grafting methods (reversed vein bypass grafts, 10%; nonreversed translocated, 13%; in situ, 16%). A normal intraoperative scan on initial imaging (n = 464 scans) or after revision (n = 67 scans) is associated with a 30-day thrombosis rate of 0.2% and a revision rate of 0.8% for duplex-detected stenosis (PSV > 300 cm/s). By comparison, 20 (21%) of 95 bypass grafts with a residual (n = 29 grafts) or unrepaired duplex stenosis (n = 53 grafts) or low flow (n = 13 grafts) had a corrective procedure for graft thrombosis (n = 8) or stenosis (n = 12); p < 0.001. Correction of residual graft defects and rescanning to document absence of residual stenosis reduced the incidence of graft problems identified on postoperative duplex surveillance [25].
The algorithm for intraoperative duplex ultrasound assessment of an infrainguinal vein bypass involves imaging the entire arterial reconstruction and classification of findings into one of four categories (Fig. 24.6and Table 24.2). Scanning is performed after completion of the bypass graft, and patency is verified by clinical inspection and pulse palpation [26]. A 10–15-MHz linear array transducer, placed in a sterile plastic sleeve containing acoustic gel, is used to scan exposed vessels and graft segments beneath the skin. Imaging of the vein graft, anastomoses, and adjacent native arteries is performed in a longitudinal plane along the vessels, beginning at the distal graft-anastomotic segment and then proceeding proximally to include the entire venous conduit and the inflow artery-proximal anastomosis graft segment. Papaverine HCl (30–60 mg) is injected into the distal vein bypass via a 27-gauge needle to augment flow, thereby increasing the diagnostic sensitivity for stenosis detection. Graft PSV is measured (60° or less Doppler angle) at the proximal and distal anastomosis, at selected sites along the graft length (high-thigh, HT; above-knee, AK; below-knee, BK; and distal graft), and from inflow and outflow arteries. Following in situ saphenous bypass, imaging for patent side branches is performed, and the absence of a side-branch flow is confirmed by the presence of a staccato waveform with distal graft occlusion.
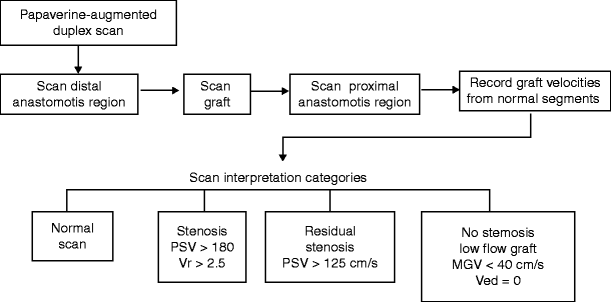

Fig. 24.6
Algorithm for intraoperative duplex scanning of an infrainguinal vein bypass. Following duplex assessment, the study is classified into one of four categories: normal, stenosis identified, residual stenosis identified, or no stenosis identified, but hemodynamic assessment of graft flow demonstrates low (PSV< 40 cm/s) velocity and high resistance (no flow in diastole, Ved = 0)
Table 24.2
Interpretation of intraoperative duplex ultrasound studies of infrainguinal vein bypasses and suggested perioperative management
Duplex scan category | Graft flow velocity(cm/s) | Peripheral vascular resistance | Interpretation and perioperative management |
|---|---|---|---|
Normal | >40 | Low | No stenosis identified and graft PSVais normal; administer dextran-40 (25 ml/h, 500 ml) and oral aspirin (325 mg/day) |
Stenosis | <40 | Low | Correct lesion and rescan graft if no residual stenosis is identified but graft PSV is low (<40 cm/s); administer heparin anticoagulation (weight based) or low molecular weight heparin (1 mg/kg sc bid), dextran-40 (25 ml/h), and oral aspirin (325 mg/day) |
PSV > 180 cm/s | |||
Vr > 2.5 | |||
Residual stenosis | >40 | Low | Rescan after 10 min to confirm no progression; administer low molecular weight heparin (1 mg/kg sc bid), dextran-40 (25 ml/h), and oral aspirin (325 mg/day) |
PSV < 180 cm/s | |||
Vr < 2.5 | |||
Low flow, no graft stenosis | <40 | High | Consider an adjunctive procedure to increase graft flow (distal arteriovenous fistula, jump/sequential graft to another outflow artery); if not possible treat as low-flow graft with an antithrombotic regimen of heparin anticoagulation, dextran-40, and aspirin (325 mg/day) |
Vein conduit stenosis is the most frequent abnormality found with intraoperative duplex scanning. Other abnormalities, in decreasing frequency, include anastomotic stenosis, platelet thrombus, and low graft flow as a result of outflow disease. Immediate repair is indicated for vein valve/anastomotic sites with PSV > 180 cm/s and a velocity ratio (Vr = PSV at lesion/PSV proximal) of >2.5 (Fig. 24.7). Velocity spectra of a high-grade stenosis (PSV > 300 cm/s) at the site of the imaging graft abnormality may represent formation of platelet thrombus. This graft segment should be replaced and a thrombolytic agent infused downstream to lyse thrombus embolized to the distal graft and runoff arteries. In vein grafts of normal (3–5 mm) caliber, a low PSV was measured in only 13 of the 626 grafts, of which 6 were to blind segments. Five of the 13 grafts failed within 90 days. Rzucidlo et al. [24] also reported a high rate of early graft thrombosis when duplex scanning demonstrated no or low (<8 cm/s) flow in diastole in the distal graft. When a high-resistance, low graft flow is identified, a careful search for a technical error should be performed, and if none is identified, perioperative heparinization or a procedure to increase graft flow (sequential bypass, distal arteriovenous fistula) should be considered.
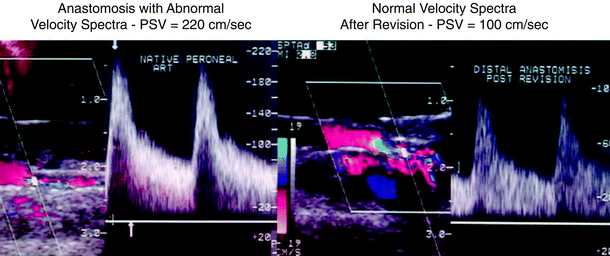

Fig. 24.7
Intraoperative color duplex scan of a residual stenosis at the distal anastomosis of a femoral-peroneal saphenous vein bypass (left). Following revision by vein patch angioplasty, repeat scanning shows normal velocity spectra (PSV< 150 cm/s)
Postoperative Graft Surveillance
Duplex ultrasound is used to confirm graft patency, identify stenotic lesions, assess their risk for producing graft thrombosis, and, if not repaired, monitor stenosis progression.
Testing should include a clinical evaluation for symptoms or signs of limb ischemia, measurement of ABI, and color duplex imaging of the entire bypass graft including anastomosis and inflow/outflow arteries. A “predischarge” scan is recommended to confirm normal functional patency, identify the presence of technical problems and the progression of a residual stenosis detected at operation, and provide baseline graft flow velocities. Upon discharge, patients should be counseled and their primary care physician notified regarding the importance and necessity of graft surveillance. Graft failure has been associated with lack of a surveillance examination within 3 months of the procedure [27]. At each graft surveillance study, patients should also be queried regarding current use of tobacco products and whether they are taking antiplatelet medications. Modifying these two factors associated with graft failure can improve long-term patency.
Testing Intervals
Factors affecting the frequency of surveillance include the type of bypass and results of the predischarge duplex scan (Table 24.3). Grafts at higher risk include those with residual stenosis, those modified during surgery, and those with low graft velocities. If the predischarge scan is normal, the first outpatient graft surveillance is performed 1 month after discharge. At this time, complete graft imaging should be possible, and if no abnormalities are identified, the next surveillance study is scheduled for 3 months later, and every 6 months thereafter if normal surveillance studies are obtained. Bypasses with low-flow, residual graft lesions or constructed of arm veins should be evaluated every 3 months for the first year.
Table 24.3
Risk stratification for graft thrombosis based on surveillance dataa
Categoryb | High-velocity criteria
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|