FIGURE 35.1. The composite flap as shown consists of a segment of the peroneal artery, peroneal vein, and a portion of the fibula with the perforating arteries supplying the overlying skin.
(From Urken ML, Sullivan MJ. Fibular osteocutaneous free flaps. In Urken ML, Cheney ML, Sullivan MJ, et al (eds). Atlas of Regional and Free Flaps for the Head and Neck Reconstruction. New York: Raven, 1995, pp 291–206, with permission.)
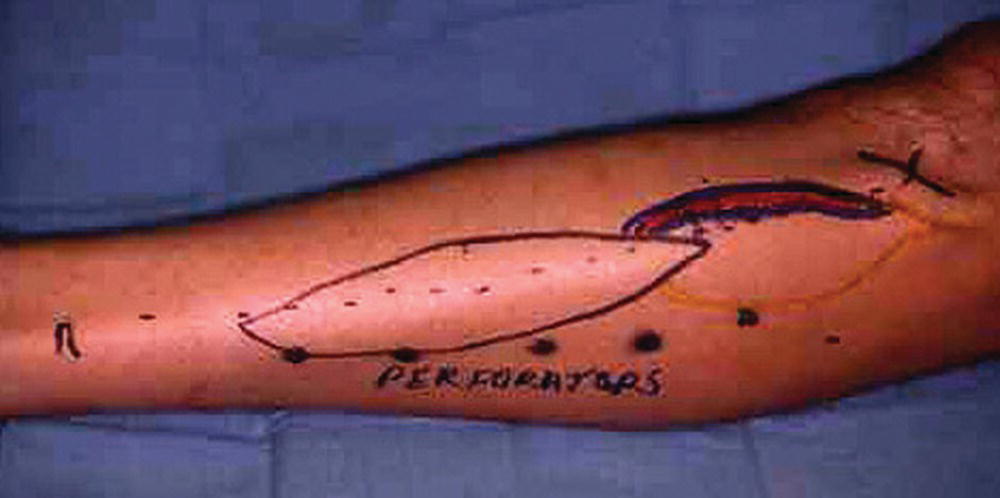
FIGURE 35.2. Preoperative marking for a fibula osteocutaneous free flap dissection. Note the location of the peroneal perforators.
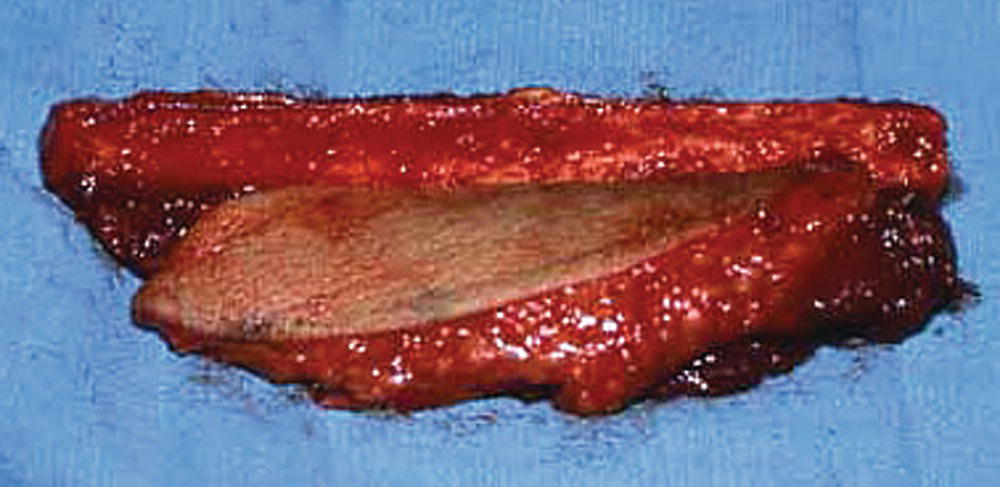
FIGURE 35.3. A harvested fibula osteocutaneous free flap.
The most feared donor site complication in fibula flap harvest is foot ischemia secondary to sacrifice of the peroneal artery. In most people, the peroneal artery does not supply a significant amount of the pedal circulation, but there are particular features of the vascular anatomy of the lower leg that may preclude the fibula from being transferred as a vascularized free flap.2,3 Because the peroneal artery is almost always present, the fibula flap has a dependable pedicle; however, its removal may jeopardize the vascular supply to the leg if the posterior tibial or anterior tibial arteries are diseased, absent, or aberrantly originating from the peroneal artery. The peroneal artery usually terminates above the ankle joint, whereas the anterior and posterior tibial arteries continue across this joint to supply the dorsal and plantar surfaces of the foot, respectively. Congenital anomalies or acquired disease of the infrageniculate vessels is estimated to result in a dominant peroneal artery in 7% to 12% of the population.4 An uncommon but important anatomic variant is that of arteria peronea magna, a congenital anomaly in which the peroneal artery is the sole vessel to the foot, with patients having normal distal pulses and no associated ischemic symptoms. This condition has been described as occurring in 0.2% to 0.9% of the population.5,6 These observations have led Urken et al3 to state that the most serious consequence of fibula flap transfer is the lack of collateral circulation to the foot, leading to ischemia following interruption of the peroneal artery.
PREOPERATIVE ASSESSMENT
Controversy exists regarding the extent of evaluation that is required in the preoperative assessment of fibula free flap donor sites. Large series have been reported without the benefit of a preoperative vascular assessment of any kind and with no adverse sequelae.1,2 Some reconstructive surgeons believe that a thorough history and physical examination should be the primary screening tools and that imaging should be used only for the small percentage of patients who have abnormal pedal pulses, a history of trauma, or symptoms suggestive of claudication.4,6 Others believe that vascular imaging is necessary in the preoperative assessment of all potential fibula free flap candidates.7,8 Many head and neck cancer patients are elderly and have a history of smoking as well as a higher prevalence of peripheral vascular disease than is found in the general population. In a survey of 206 vascular surgeons in the United Kingdom, 88% of respondents indicated that not performing presurgical vascular imaging bordered on clinical negligence.9 Our opinion is that it is essential that patients be safely and comprehensively evaluated for vascular disease or significant anatomic variants before surgery. Defining the length and branching pattern of the peroneal artery preoperatively also provides the surgeon with a “roadmap” that aids and shortens the time required for peroneal artery dissection. The viability of such flaps is entirely dependent on the integrity of their vascular supply, making the detection of peroneal artery disease or anomaly of paramount importance.10
Standard catheter angiography has long been the “gold standard” for the evaluation of lower extremity vascular patency, allowing high-resolution imaging of the trifurcation vessels, including assessment for the presence of significant stenoses or congenital branch anomalies. Numerous investigators have reported angiographically detected abnormalities that altered the operative plan in up to 25% of cases.8,11 However, this technique is invasive and involves considerable ionizing radiation exposure for the patient, and it also suffers from the shortcomings of high cost and potential morbidities (hematoma, thrombosis, vessel injury, and contrast reactions). Less invasive tests, such as magnetic resonance angiography (MRA) and computed tomography angiography (CTA), have been reported to be equally effective in peripheral vascular mapping without arterial catheterization. MRA offers the capability of reliable noninvasive vascular imaging in the absence of ionizing radiation exposure and without the use of iodine-based contrast media.12 Furthermore, MRA has the advantage of costing less than one-half as much as conventional angiography. However, MRA is problematic for the claustrophobic patient and requires special hardware (surface coil) to enhance imaging. CTA involves considerable ionizing radiation exposure, administration of iodine-based contrast media, and potential for nonvisualization of the trifurcation vessels due to the presence of “blooming” artifact from adjacent calcified plaque.13,14
These limitations have prompted the application of less invasive and less expensive imaging techniques in the routine preoperative assessment of patients being considered for fibula free flap procedures. Significant interest has focused on the use of color-flow duplex (CFD) ultrasound in this clinical setting because of its accuracy in mapping the vascular anatomy and relative cost savings when compared with both angiography and MRA.7,10,15 CFD combines B-mode imaging, color-flow Doppler, and Doppler spectral waveform analysis to noninvasively assess vessel patency and blood flow.16–18 Hatsukami et al19 and Kerr and Bandyk20 have demonstrated the ability of CFD to accurately examine the lower extremity vasculature and diagnose arterial occlusive disease. CFD resolution of flow in vessels as small as 1 mm in diameter has also been demonstrated. Aly et al21 reported a sensitivity of 92% and specificity of 99% for duplex sonography compared with conventional angiography in the evaluation of lower limb arterial occlusive disease. Moreover, as discussed in Chapter 36, vascular mapping of the rectus abdominis muscle by CFD prior to breast reconstruction has been valuable for incision planning.22
These facts served as a catalyst to design a clinical protocol to study the effectiveness of CFD evaluation prior to fibula free flap harvest. Examination of the lower extremity vasculature with CFD can be completed within 60 minutes. Doppler sonography affords the additional capability to map the location and extent of cutaneous perforators from the peroneal artery, an important determinant of skin-paddle viability in the osteocutaneous fibula flap. Futran et al15 applied this technique to the assessment of 38 patients in preparation for fibula free flap harvesting, and the results of the vascular evaluation precluded flap transfer in 4 (10.5%). In a similar study, Futran et al23 further described the use of the ankle-brachial index (ABI) in the preoperative evaluation of potential fibula free flap candidates. They found good correlation between an ABI of less than 1.0 and subsequent arterial occlusive disease confirmed by angiography. However, they also found an unacceptable number of patients with an ABI greater than 1.0 who had arterial disease. Thus, they concluded that an ABI of less than 1.0 can exclude lower extremities as donor sites, but a lower extremity with an ABI of greater than 1.0 requires further evaluation by CFD.
VASCULAR LABORATORY EVALUATION
Lower extremity arterial duplex scanning is covered in detail in Chapter 12, and lower extremity venous scanning is described in Chapter 19. The techniques and basic concepts of these examinations are applied to the preoperative vascular laboratory assessment prior to fibula free flap transfer in order to achieve the following goals.
1. Rule out significant obstructions in the lower extremity arteries.
2. Identify significant anatomic variants (branching patterns) and arterial disease of the posterior tibial and anterior tibial arteries.
3. Provide a roadmap for the surgeon, documenting anatomy and patency of the peroneal artery.
4. Obtain the size and number of peroneal artery perforators, marking their locations on the leg.
5. Rule out chronic or acute deep vein thrombosis in the popliteal and tibial veins with special attention to the peroneal veins.
The testing algorithm described in this chapter was developed in the 1990s by Dr. Futran et al in collaboration with the D.E. Strandness, Jr, Vascular Laboratory.15 Since that time, the overall image quality and color Doppler sensitivity of duplex ultrasound instruments has improved significantly. The original Futran algorithm was subsequently modified to include Doppler waveforms from the tibial arteries at the ankle. This provides identification of arterial occlusive disease in patients with calcified and noncompressible tibial arteries. We believe these improvements have reduced the time required to perform the examination and the operator dependence to obtain accurate results. In addition, lower extremity arterial pressures and Doppler waveforms provide a simple screening method that other forms of diagnostic testing do not offer.
UNIVERSITY OF WASHINGTON PROTOCOL
Preparation
- Allow at least 1 hour and up to 2 hours to perform the examination and report the findings.
- Schedule the examination within 1 week of surgery and instruct the patient to use a permanent ink pen to maintain the markings if the ones made during the examination begin to fade prior to surgery.
- Perform the examination with the patient supine for duplex assessment of the anterior tibial and posterior tibial arteries. The popliteal artery may be better visualized with the patient in the prone position. The peroneal artery and perforators are easily visualized with the patient in the lateral decubitus position.
Equipment
- Duplex ultrasound system (B-mode, color Doppler, and spectral waveform analysis)
- Midrange transducer (4 to 10 MHz)
- Blood pressure cuffs (10 cm wide for the ankle and 12 cm wide for the arms; two of each)
- Sphygmomanometer
- Permanent marking pen
- Measuring tape or ruler
Ultrasound System Presets
- Color Doppler settings (mean flow) are adjusted to approximately 30 cm/s for the popliteal artery and 15 cm/s for the tibial arteries. The range may be reduced for lower flow velocities in the perforator arteries.
- Doppler settings for arterial spectral waveforms are optimized for peak flow velocities ranging from 60 to 140 cm/s in the popliteal artery and 25 to 110 cm/s in the tibial arteries. Angle correction is utilized for all arterial velocity measurements, including the perforators. This requires an angle of 60 degrees or less between the incident Doppler ultrasound beam and the arterial wall.
- Doppler settings for venous spectral waveforms are lower than for arterial spectral waveforms and are optimized for velocities ranging from 10 to 20 cm/s.
EXAMINATION TECHNIQUE
Step 1. Rule Out Atherosclerotic Disease in the Lower Extremity with the ABI and Doppler Waveforms
Documentation of the ABI is the first step in the modified Futran algorithm shown in Figure 35.4. As discussed in Chapter 13, in most patients, the ABI measurement will detect any significant arterial occlusive disease in the lower extremity. Systolic pressures are recorded with Doppler using all three tibial arteries (anterior tibial/dorsalis pedis, posterior tibial, and peroneal) at the level of the ankle. When different pressures are obtained in the various tibial arteries, the highest value should be used to calculate the ABI. A ratio of this ankle pressure to the highest brachial systolic pressure of less than 1.0 indicates the presence of lower extremity arterial obstruction. If the ABI is clearly abnormal (<0.95), it is our custom to contact the referring provider, and the examination of that limb is likely to be discontinued. Calcification of the vessel walls is reported when arterial pressures cannot be measured at the ankle owing to vessel noncompressibility (ankle pressures >250 mm Hg) or when the ABI is >1.40. In these cases, we rely on Doppler waveform analysis of flow in the tibial arteries at the ankle. If the Doppler waveforms are normally multiphasic (Fig. 35.5A) as opposed to abnormally monophasic (Fig. 35.5B), and the ABI is ≥1.0 but ≤1.40, the protocol proceeds to the next step.
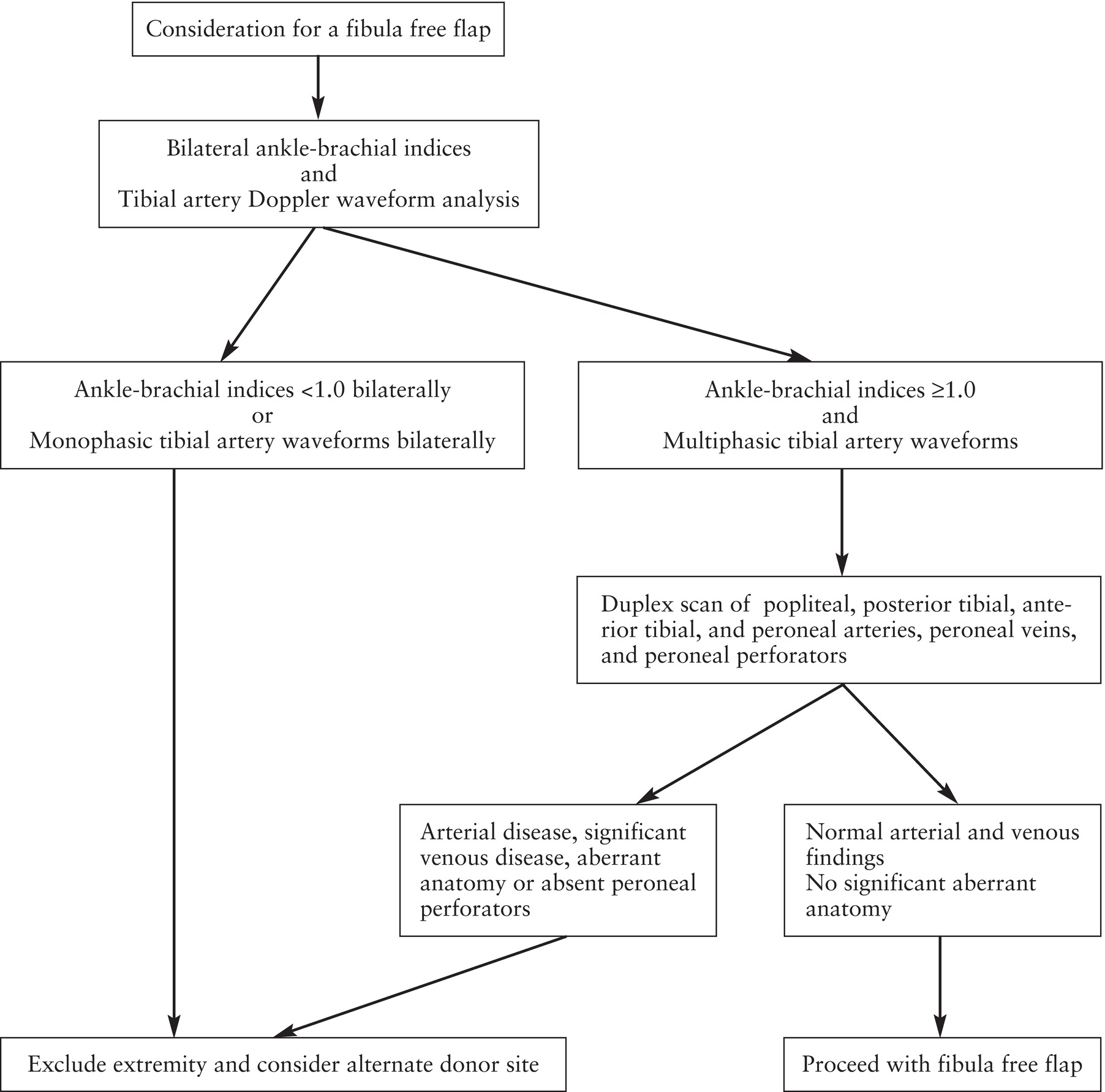
FIGURE 35.4. Algorithm for preoperative assessment of fibula free flap donor sites.
(Modified from Futran ND, Stack BC Jr, Zachariah AP. Ankle-arm index as a screening examination for fibula free tissue transfer. Ann Otol Rhinol Laryngol 1999;108:777–780.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


