Duplex diagnosis
Endovascular
Surgery
Aortoiliac lesions
Focal stenosis
Stent
Endarterectomy/reconstruction
Diffuse disease
Balloon angioplasty
Aortofemoral bypass
Stent
Femorofemoral bypass
Aortic or iliac aneurysm
Stent graft
Surgical reconstruction
Infrainguinal lesions
Femoropopliteal segment
Common femoral
Balloon angioplasty
Endarterectomy/reconstruction
Profunda femoris
Balloon angioplasty
Endarterectomy/reconstruction
Superficial femoral
Balloon angioplasty
Bypass reconstruction
Atherectomy
Stent
Popliteal-tibial segment
Balloon angioplasty
Bypass reconstruction
Atherectomy
Popliteal aneurysm
Stent graft
Exclusion with bypass
Pseudoaneurysm
Iatrogenic
USGTIa
Surgical repair
Graft
Stent graft
Surgical reconstruction
Arteriovenous fistula
Embolization
Surgical repair
Vein graft stenosis
Balloon angioplasty
Surgical revision
Certification of the vascular laboratory serves to communicate the laboratory’s commitment to delivering valued studies at the highest quality and provides a measurable standardization that can be reproduced regionally. The vascular lab should involve physicians and technologists credentialed and experienced with all facets of scanning and interpretation of vascular testing. Contemporary vascular disease evaluation and management mandates duplex scanning be an integral part of diagnostic and therapeutic algorithms for screening, intervention, and surveillance of PAD. Agency accreditation provides the vascular laboratory with a voice to secure fair reimbursement and guidelines for conducting laboratory services.
Preintervention Arterial Testing
Arterial testing should be individualized taking into account current clinical signs and symptoms (e.g., dependent rubor, ulceration, gangrene) and any recent PAD surveillance scans. Symptomatic lower extremity occlusive disease should have limb pressures measured at one or more levels in combination with Doppler or plethysmographic (pulse volume) waveform analysis. Measurements of ankle-brachial systolic pressure index (ABI) and digit systolic pressures can adequately characterize the severity of PAD (Fig. 23.1). Toe systolic pressures are especially helpful in diabetics in whom calcified, incompressible tibial vessels produce erroneously high ABIs (>1.3). Atypical presentations of exertional leg pain, especially in patients with an abnormal ABI (<0.9), should be considered for exercise treadmill testing in order to exclude nonvascular conditions that may be responsible for lower extremity claudication-like pain. Other indications for peripheral arterial testing include absent pulses, disabling claudication, ulceration, gangrene, or rest pain. Any of these findings should prompt a color duplex examination to characterize disease location, extent, severity, and morphology (atherosclerosis, aneurysm). Duplex testing can also identify other relevant concomitant vascular conditions such as renal artery stenosis, aneurysm development, or venous thrombosis.
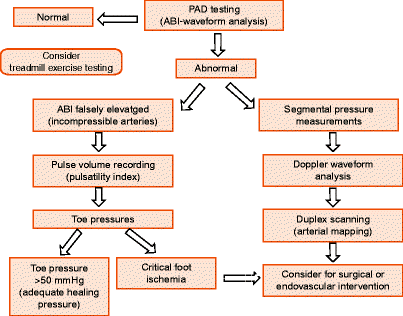

Fig. 23.1
Vascular laboratory evaluation of peripheral artery disease (PAD) by ankle-brachial index (ABI), Doppler waveform analysis, pulse volume recordings (PVR), digit pressures, exercise testing, and duplex arterial mapping to develop treatment plan
Duplex scanning is particularly helpful in stratifying the level of occlusive disease (e.g., aortoiliac, femoropopliteal, popliteal-tibial, or multilevel disease segments). Additional clinical applications for duplex scanning include:
Evaluation of asymptomatic patients with abnormal (<0.9) ABIs to ascertain intervention by endovascular (e.g., percutaneous angioplasty or stenting) or surgical bypass
Exclusion of occult inflow (aortoiliac) disease in patients requiring femoral-distal bypass grafting
Evaluation of specific diseased arterial segments (outflow atherosclerosis or isolated stenoses) visualized on diagnostic arteriography whose hemodynamic significance is not clear
Alternative imaging to reduce angiographic contrast exposure in patients with renal insufficiency
Identification of atheroembolism or acute arterial thrombosis (i.e., blue toe syndrome)
Assessment of percutaneous catheterization sites for pseudoaneurysm or arteriovenous fistula
Evaluation of vascular injury associated with blunt and penetrating trauma
Surveillance of surgical bypass grafts or reconstructions, endovascular interventions, or dialysis access for stenosis caused by myointimal hyperplasia, fibrosis, or atherosclerosis
The accuracy of duplex scanning is sufficient to permit arterial mapping analogous to contrast arteriography in body regions accessible to diagnostic ultrasound imaging. Classification of lesion severity is based on the same physical principles that apply to the duplex evaluation of the cerebrovascular, renal, and mesenteric circulations. Compared to arteriography, the “gold standard” for peripheral arterial imaging, duplex scanning has a diagnostic accuracy of >80% for the detection of a >50% diameter reducing stenosis or occlusion (Table 23.2) [1, 2, 4, 7–9]. Diagnostic accuracy decreases when multilevel disease is present. However, in the absence of multilevel disease, diagnostic accuracy exceeds 90% for the detection of high-grade stenosis or occlusion involving iliac, femoral, popliteal, or tibial arterial segments. Additionally, several centers have conducted small prospective blind trials comparing duplex imaging to contrast arteriography for planning infrainguinal reconstructions for occlusive disease. These studies indicated that duplex imaging was equal to angiography in predicting suitable distal bypass with confidence intervals in the range of 95% [10, 11]. In more than 50% of patients with symptomatic PAD, duplex scanning will identify disease amenable to endovascular therapy [9, 13]. Duplex imaging to plan infrainguinal bypass procedures for occlusive disease has also been studied in prospective trials with results compared to contrast angiography. Patient outcomes (limb salvage, graft patency) were similar indicating that the clinical accuracy of duplex testing to select appropriate inflow-outflow anastomotic sites for lower limb arterial bypass was equivalent to angiography [10, 11]. Whether an arterial lesion is suitable for endovascular repair generally depends on specific anatomic characteristics. Duplex findings of Trans-Atlantic Inter-Society Consensus (TASC) category A or B lesions indicate endovascular intervention is the preferred treatment (Table 23.3). Technical success rates in excess of 95% can be achieved with clinical results similar to surgical reconstruction. Category C lesions (>4-cm-long calcified stenosis, multilevel disease, 5–10-cm-long chronic occlusions) may also be amenable to endovascular repair depending on the experience of the vascular surgeon. Endovascular treatment of category D (diffuse stenosis, >10-cm occlusions) lesions is not associated with outcomes comparable to “open” surgical repair or bypass grafting [11, 12, 14]. In applying duplex scanning to patient evaluation, the intent is to characterize the extent and severity of occlusive disease to permit a clinical decision regarding intervention options.
Table 23.2
Diagnostic accuracy (sensitivity/specificity) of color duplex ultrasonography compared with diagnostic contrast angiography for hemodynamically significant lesions
Author | Iliac artery | Common femoral artery | Deep femoral artery | Superficial femoral artery | Popliteal artery | Tibial artery |
|---|---|---|---|---|---|---|
Crossman et al.a | 81/98 | 70/97 | 71/95 | 97/92 | 78/97 | 50/8 |
Moneta et al.a | 89/99 | 76/99 | 83/97 | 87/98 | 67/99 | 90/2 |
Allard et al.a | 89/99 | 36/98 | 44/97 | 92/96 | 37/92 | – |
Kohler et al.a | 89/90 | 67/98 | 67/81 | 84/93 | 73/97 | – |
Aortoiliac | Femoropopliteal | Tibial | ||||
Hingorani et al.b | 81/84 | 75/90 | 43/65 | |||
Table 23.3
TASC (Trans-Atlantic Inter-Society Consensus) classification of lower limb arterial occlusive lesions suitable for percutaneous transluminal angioplasty (PTA)
Site of arterial lesiona | ||
|---|---|---|
Category | Aortoiliac | Femoropopliteal |
A | <3 cm focal stenosis | <3 cm focal stenosis or occlusion |
B | Single stenosis 3–10 cm | 3–5 cm single stenosis or occlusion |
Unilateral CIA occlusion | Heavily calcified lesions ≤3 cm | |
Two stenosis <5 cm | Lesions with tibial occlusion | |
Multiple lesions <3 cm | ||
C | Unilateral EIA occlusion not involving CFA | Single stenosis or occlusion >5 cm |
Unilateral EIA stenosis extending into CFA | Multiple lesions 3–5 cm | |
Bilateral stenosis 5–10 stenosis | Multiple lesions >5 cm | |
Bilateral CIA occlusion | ||
D | Iliac stenosis with aortic or iliac aneurysm | Complete CFA or SFA and popliteal or proximal tibial vessel occlusion |
Diffuse stenosis >10 cm of CIA, EIA, CFA | ||
Unilateral occlusion CIA and EIA | ||
Bilateral EIA occlusion | ||
Continued technological advances have improved the imaging quality of color flow Doppler in a manner analogous to arteriography. The classification of occlusive lesions is based on the same general principles that apply to the duplex evaluation of other arterial circulatory systems (e.g., cerebrovascular, mesenteric, renal). When duplex scanning has been used in the evaluation of symptomatic lower limb atherosclerotic disease, approximately 45% of patients have lesions suitable for treatment with endovascular techniques [9, 13]. Whether a diseased arterial segment is suitable for endovascular intervention depends on the specific characteristics of the lesion. In the lower limb, duplex findings of category one or two lesions based on the Society of Cardiovascular and Interventional Radiology guidelines indicate endovascular intervention is a treatment option (Table 23.3) [12]. Technical success rates in excess of 95% can be achieved with clinical results similar to surgical reconstruction. Category three lesions (>4-cm-long calcified stenosis, multilevel disease, 5–10-cm-long chronic occlusions) are also amendable to endovascular procedures. While short-term primary patency rates are comparable to surgical bypass grafting, mid- and long-term patency rates remain below that of surgical reconstruction.
Color Duplex Peripheral Arterial Examination
For most examinations, 30–45 min should be allotted. The vascular examination room should be kept warm (75–77°F) to avoid vasoconstriction. The patient should be instructed not to eat within 6 hours of the examination in order to reduce abdominal gas in the event that aortoiliac imaging is required. Abdominal imaging begins with a 3–5-MHz phased array transducer with the evaluation of the infrarenal aorta at the level of the renal artery origins and moves caudally toward the iliac arteries. As the exam is continued to the inguinal ligament at the level of the femoral artery, the transducer frequency is increased to a 5–7-MHz probe. Multiple scanning windows may be required for complete insonation/imaging of the pelvic and infrainguinal circulation due to imaging limitations such as obesity (vessels >15 cm deep), bowel gas, large limbs, edema, surgical wounds, ulcers, joint contractures, small vessels, and vessel calcification. Aortic diameter is documented as the technologist moves distally evaluating the iliac circulation, followed by the common femoral, deep femoral, superficial femoral, popliteal, and mid to distal tibial vessels. B-mode imaging can be used to measure diameter and document plaque character or stenosis. Color Doppler permits rapid location of sites of turbulence by lumen narrowing, color map aliasing, color flow jets, and occasionally a tissue bruit. Identification of vessel branching, collateral circulation, aneurysmal change, and occlusive disease as well as sampling blood flow patterns are important components of duplex imaging. Because occlusive lesions have a tendency to develop at specific sites, scanning should particularly be focused on these areas especially when proximal-to-distal changes in velocity waveform configuration are recorded (Table 23.4) [6, 7]. In order to adequately grade the severity of stenosis, a center-stream Doppler angle corrected to 60° with pulsed Doppler spectral analysis is carried out proximal to, at the site of maximum flow disturbance, and distal to the site of stenosis. Attention to changes in velocity waveform (pulsatility) and measurement of peak systolic and end-diastolic blood flow velocities are recorded. Identification of luminal narrowing, plaque character, color map aliasing (turbulent flow), color flow jets, and tissue bruits should be documented if present (Fig. 23.2). Doppler velocity spectra from the distal tibial and pedal arteries are assessed and should aid in correlating waveform pulsatility and peak systolic velocity with measured ABI (Fig. 23.3). Again, this correlation is especially important if heavily calcified or incompressible vessels are present. An ABI value of >1.3 suggests the presence of noncompliant or calcified vessels. Assessment of pulsed Doppler spectra at all stations of the extremity – common femoral, superficial femoral, popliteal, and tibial vessels – allows for comparison of pulsatility index and acceleration time between arterial segments.
Table 23.4




Most common patterns of lower limb atherosclerosis or location of stenosis after infrainguinal endovascular or surgical intervention
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


