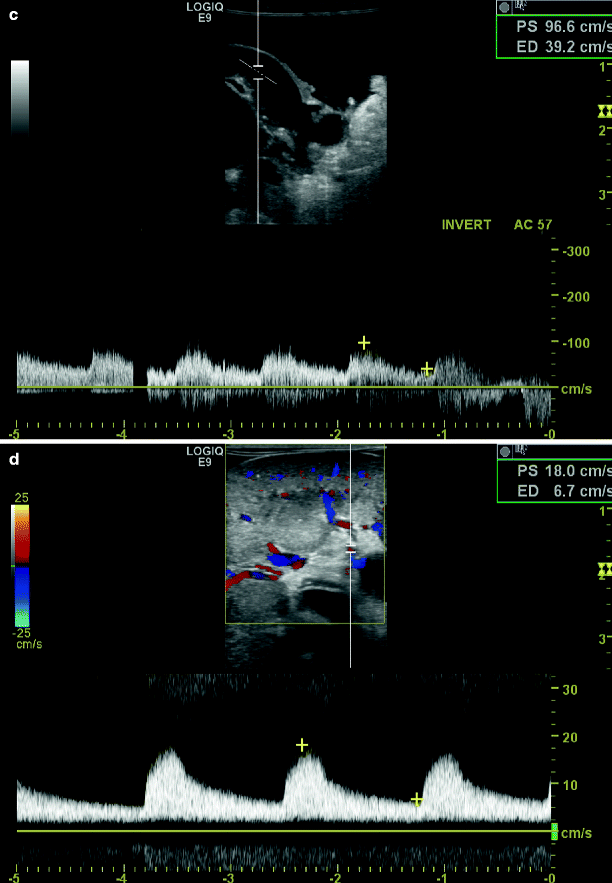
Fig. 48.1
Intraoperative duplex sonography of main renal artery prior to repair in longitudinal (a) and transverse view (b). Post-repair duplex (c) with corresponding spectral waveform analysis and renal parenchymal signals (d)
Intraoperative studies are performed with a 5 MHz linear array probe with Doppler color flow. The probe head is placed in a sterile plastic sheath containing acoustic gel. The operative field is flooded with warm saline and scanned images are first obtained in the longitudinal projection. Care is taken to image the aorta, the renal artery origin and the entire renal artery from origin to hilum. All defects seen in longitudinal projection are imaged in transverse fashion to confirm their anatomic presence and estimate the contribution to luminal narrowing. Doppler samples are then obtained proximal and distal to the imaged defects in longitudinal projection to determine their potential contribution to flow disturbance. Finally, intrarenal Doppler signals from the interlobar and arcuate branches are obtained in the upper, mid portion, and lower pole of the kidney.
The criteria for intraoperative defects creating ≥60% diameter reduction are similar to surface renal duplex sonography studies (Table 48.1). These criteria have been validated in an animal model of graded renal artery stenosis and compared with pre- and post-operative angiography in more than 100 patients. Unlike surface duplex sonography in which the Doppler sample is large relative to the renal artery diameter, the Doppler sample can be accurately positioned within the mid-center of arterial flow. However, at this mid-center location at least moderate spectral broadening is inherent to the Doppler spectrum after open repair in the absence of anatomic defect.
Table 48.1
Intraoperative Doppler velocity criteria for B-scan defects
Defect | Criteria |
|---|---|
<60% of diameter-reducing RA defect | RA-PSV from entire RA < 1.8 m/s |
>60% diameter-reducing RA defect | Focal RA-PSV > 1.8 m/s and distal turbulent velocity waveform |
Occlusion | No Doppler-shifted signal from renal artery B-scan image |
Inadequate study for interpretation | Failure to obtain Doppler samples from entire main renal artery |
During intraoperative renal duplex sonography, the interaction between surgeon and vascular technologist is important. Both B-scan images and velocity estimates from spectral data are enhanced by the participation of a vascular technologist. Although the surgeon is responsible for manipulation of the probe head to ensure optimal B-mode images at likely sites of technical error, the power and time adjustments to minimize artifact are best made by an experienced technologist. Close cooperation is required to obtain complete pulse Doppler sampling and estimate velocities associated with B-scan defects. Although often overlooked, the participation in intraoperative studies enhances the technologist’s subsequent ability to obtain satisfactory surface renal duplex images during follow-up surveillance.
In over 500 intraoperative renal duplex sonographies after renal artery reconstruction, the average time for intraoperative scan was less than 5 min [10]. Complete B-scan and Doppler derived velocity data were obtained in over 98% of cases. Renal duplex sonography was considered normal, free of any B-scan defect in 77% of cases while B-scan defects were present in 23%. Eleven percent of defects had Doppler velocity estimates exceeding 1.8 m/s with post-stenotic turbulence and these were defined as major. These major defects underwent immediate operative revision and in each case a significant defect was discovered and corrected. Successful revision was verified by a second intraoperative duplex study. In follow up, 97% of these open operative repairs have maintained primary patency without recurrent stenosis at a 5-year follow up. Neither uncorrected minor B-scan defects nor corrected major B-scan defects have correlated with subsequent failure of open repair.
B-scan defects designated as major or minor by Doppler velocity criteria provide accurate information to guide intraoperative revision. There are, however, special circumstances that deserve comment. Intraoperative duplex study after renal repair may occasionally demonstrate peak systolic velocity that exceeds criteria for critical stenosis in the absence of anatomic defect. In these circumstances, peak systolic velocity is elevated uniformly throughout the repair with no focal velocity change and no distal turbulent waveform. Intraoperative studies of this type are most commonly encountered after renal artery repair in children and young adults for non-atherosclerotic disease. Increased peak systolic velocity can also be observed in transition from main renal artery to segmental renal vessels after branch renal artery repair. In the absence of the defect, no distal turbulent waveform will be observed. Finally a renovascular repair to a solitary kidney frequently has elevated velocities throughout without evidence of turbulence.
Other special considerations concern diastolic features of the Doppler spectrum in the absence of technical error. An abnormal diastolic spectrum may be observed after revascularization of chronic renal ischemia reflecting an increased vascular resistance in response to reperfusion. The spectrum may demonstrate brief acceleration times and virtually no diastolic flow. These changes reflect a reactive vasospasm, which can be defined by interarterial administration of 30 mg papavarine. The Doppler spectral signature characteristic for renal artery flow will normalize within 2–3 min (Fig. 48.2).
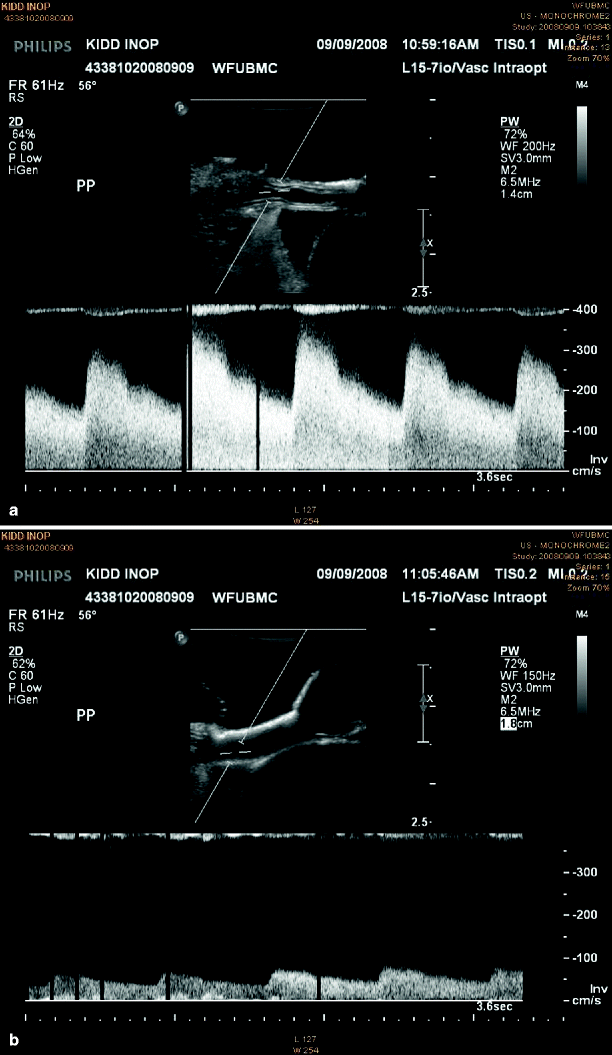

Fig. 48.2
Intraoperative duplex sonography depicting B-mode and spectral waveform analysis of main renal artery spasm pre (a) and post (b) papaverine injection
The rates of primary patency of open renal artery repair without recurrent stenosis are estimated in Fig. 48.3. This patency rate was achieved after revision of major B-scan defects in 12% of repair [11]. Although a disproportionate number of major defects were observed after transaortic endarterectomy, when revision was directed by intraoperative completion duplex, equivalent patency was observed among different reconstructive techniques. The significance of primary patency after open operative repair is profound. Whether by restenosis or occlusion treated by either reconstruction or nephrectomy, failed open operative renovascular repair is associated with an increased risk of eventual dialysis dependence, which is significant and independent of other risk factors [11].
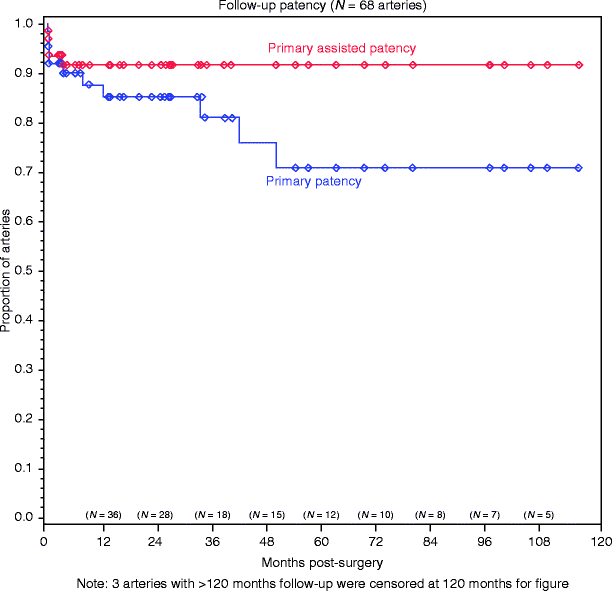

Fig. 48.3
Product-limit estimates of primary patency (blue) and primary-assisted patency (red) in 68 arteries. The standard error of the estimate does not exceed 10% (Modified from Crutchley et al. [11]. With permission from Elsevier)
Renal Duplex Sonography After Open Operative Repair of Renovascular Disease
Anatomic success of open operative renovascular repair is often assumed on the basis of a favorable blood pressure response. However, a favorable blood pressure response can occur after graft thrombosis and renal infarction, the equivalent of nephrectomy. Alternatively, a favorable blood pressure response can occur when renal perfusion is altered sufficiently to impair the renin-angiotensin system yet leaves residual lesions that may represent technical failure. For these reasons, follow-up renal angiography was for many years the method of choice for follow-up surveillance. Fortunately, our experience with percutaneous renal duplex after both open operative repair and percutaneous intervention has proved a valid substitute to assess the technical success or failure of intervention.
For post-operative surveillance, patients are fasted overnight to minimize the bowel gas interference. B-scan images and Doppler shifted signals are first obtained in a supine position with either a 2.25 MHz phased ray probe or 4/2.25 MHz curved ray probe each with Doppler color flow capability. Positioned in the abdominal midline just below the xiphoid process, the sagittal B-scan image of the upper aorta defines the origin of the visceral vessels. At this level, a center stream aortic velocity may be estimated. Using the origin of the superior mesenteric artery and the left renal vein as references, the native origin of each main renal artery can usually be defined in transverse projection during peak inspiration. Knowledge of the operation type is valuable to define suprarenal or infrarenal origins of bypass grafts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


