Left,Type I endoleaks after endovascular aneurysm repair (EVAR). Right,Type II endoleaks.
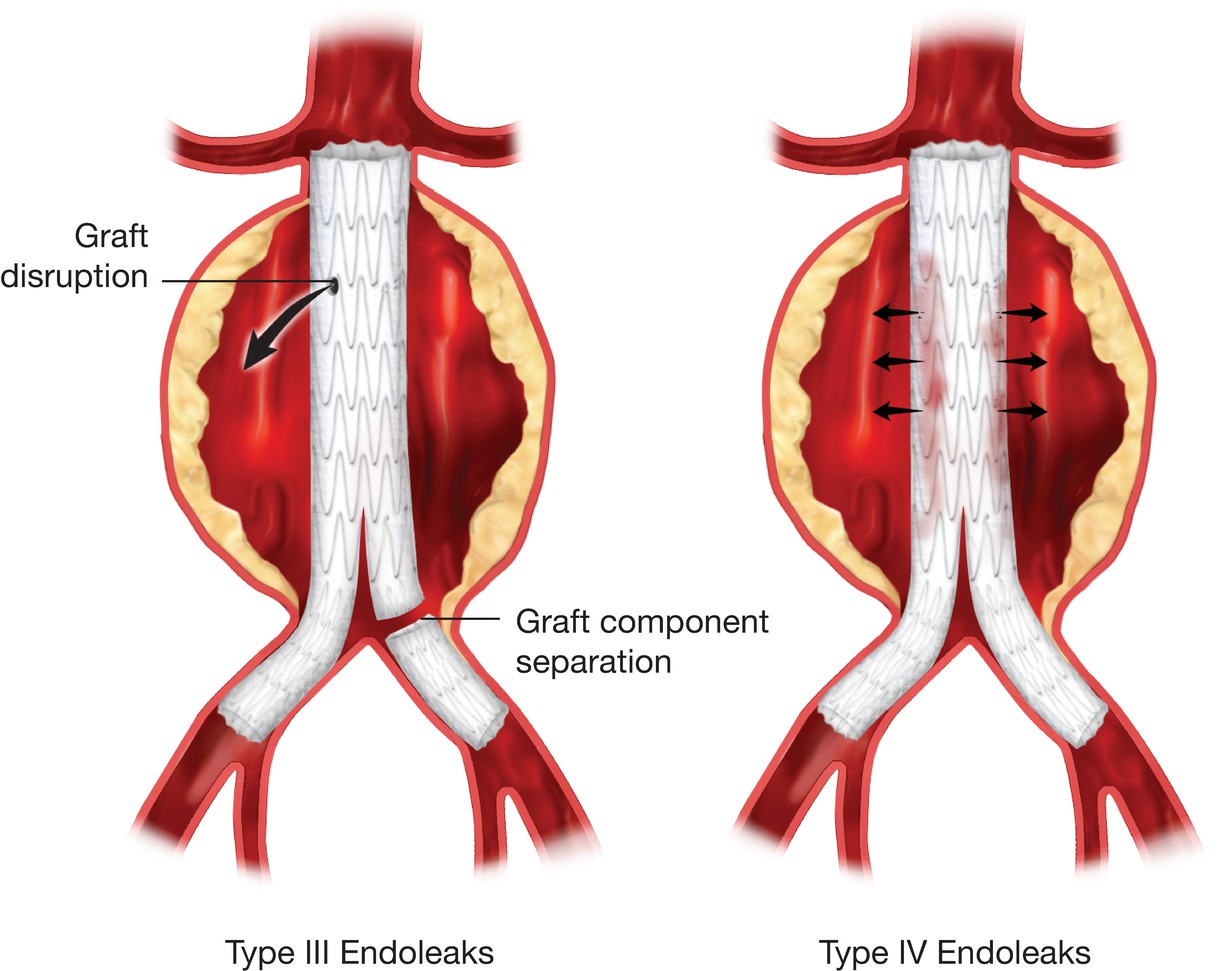
Left,Type III endoleaks after EVAR. Right,Type IV endoleaks.
A type II endoleak results from retrograde flow into the aortic sac from aortic branches such as one or more lumbar arteries, the inferior mesenteric artery, or other collateral vessels (see Fig. 16.1, Right). Type II endoleaks are relatively common and have been observed in 27% to 37% of EVAR cases.11 Some have subcategorized type II endoleaks into type IIa, indicating flow from one branch vessel, and type IIb, representing more than one branch vessel involvement.11 Others have described a type IIa endoleak as originating from the inferior mesenteric artery and a type IIb as originating from a lumbar artery. To avoid confusion, it may be better to report the presence of a type II endoleak and specifically describe the branch vessel(s) responsible. Most type II endoleaks can be followed without intervention, because many will resolve spontaneously by thrombosis.14 Interventions such as coil embolization or vessel ligation are performed when the type II endoleak is associated with evidence of aneurysm sac enlargement or failure of a large aneurysm sac to shrink over time.
A type III endoleak originates from a structural failure of the stent graft, such as a fabric tear or ineffective seal between stent graft components; these occur in less than 3% of cases (see Fig. 16.2, Left).12 Although rare, the presence of a type III endoleak is an indication for intervention by either endovascular revision or open repair because the aneurysm sac is exposed to systemic arterial pressure, and the risk of aneurysm rupture is similar to that with a type I endoleak (or an unrepaired aneurysm). Type III endoleaks can also be subcategorized into type IIIa, indicating device fabric disruption or holes; type IIIb, separation of modular component devices or junctions; and type IIIc, representing suture holes in the fabric of the graft.11
The final endoleak classification is type IV, which is a consequence of graft porosity (see Fig. 16.2, Right). Type IV endoleaks have a reported incidence of about 5%13 but are generally self-limited, resolving quickly as fibrin seals the graft material. Type IV endoleaks are infrequently encountered with the newer generations of endografts that have materials with decreased porosity covering the stents.
The term endotension may also be used in conjunction with a post-EVAR evaluation and has been referred to by some as a type V endoleak.31 Endotension represents pressure in the aneurysm sac or sac enlargement without an identifiable endoleak. Some of these cases are likely associated with endoleaks that are not visualized with the imaging studies performed. Other less likely causes of endotension include a seroma pressurizing the aneurysm sac, pressure transmission from the thrombus around the stent graft, or graft infection.31
Imaging Techniques
Although CT angiography is commonly used for post-EVAR surveillance and detection of endoleaks, this approach has a number of disadvantages, including the use of nephrotoxic contrast agents, patient exposure to ionizing radiation, and cost.17 Furthermore, certain technical factors can limit the accuracy of CT angiography, such as timing of the contrast bolus, the width of image slices, and interobserver variability.19 Pre- and postcontrast studies, typically with multidetector CT, are needed to distinguish between vessel wall calcification and endoleaks, although newer dual-energy CT systems can better differentiate calcium from contrast. It has been estimated that 33% to 65% of post-EVAR cost is secondary to the necessary radiologic studies, which may include lifelong surveillance with CT imaging.30,32
Digital subtraction angiography is usually performed to confirm suspected cases of endoleaks detected by other imaging methods. The feasibility of angiography as a surveillance tool post-EVAR is hindered by its invasiveness, exposure to radiation, and the use of nephrotoxic contrast agents. Furthermore, improper technique or the timing of an angiography contrast injection might result in an endoleak being overlooked.
Duplex Ultrasound
Duplex ultrasound is currently the preferred method for routine diagnosis and surveillance of unrepaired AAAs, as discussed in Chapter 15. In recent years, duplex ultrasonography has also evolved as an effective tool for post-EVAR surveillance. Duplex ultrasound can be used to measure the size of the residual aneurysm sac, detect endoleaks, and evaluate hemodynamic changes or stenosis in the outflow graft limbs and aortic branch vessels. Patency of the renal arteries, which can be compromised by graft fabric impingement, proximal fixation components, or proximal device migration, can be evaluated with duplex ultrasound. With enhanced resolution and better imaging technology, the ability to identify endoleaks with duplex scanning has also improved.23
The routine use of post-EVAR duplex ultrasound for surveillance has numerous advantages. Patients with renal insufficiency benefit from avoidance of repeated exposure to nephrotoxic contrast agents with CT imaging. During long-term follow-up, it has been shown that the risk for a decline in renal function is significantly greater after EVAR than after open aneurysm repair in patients with or without preexisting renal insufficiency.33,34 The ability to eliminate the use of ionizing radiation and its lower cost make abdominal duplex ultrasonography an attractive alternative. A financial analysis of aortic graft surveillance estimated the cost of a CT angiogram at $2779 per study, in comparison to the cost of an abdominal aortic duplex ultrasound at $525 per study.17 Even with the additional cost of routine abdominal radiographs needed to confirm the position of the stent graft, Bendick and colleagues17 estimated an average 3-year saving of more than $16,000 per patient followed up with duplex ultrasound rather than CT imaging after EVAR. Other potential benefits include wider availability and portability and the noninvasive nature of the procedure. However, duplex scanning may not be technically satisfactory in every case. Visualization of the aorta and the iliac vessels can be limited by the presence of overlying bowel gas, body habitus, ascites, and vessel tortuosity. Nevertheless, experience has shown that post-EVAR duplex ultrasound is an effective modality for surveillance and evaluation of aneurysm size and the presence of endoleaks.15–17,20,22–25,35,36
Duplex ultrasound is an accurate measurement tool for aortic aneurysm size. Aneurysm diameter has been shown to correlate within 5 mm between CT and duplex ultrasound in 70% to 92% of the cases.16,20,36 Raman and associates36 performed a retrospective review of 281 patients comparing color-flow duplex ultrasound and CT imaging for post-EVAR surveillance. Routine abdominal radiographs were used to assess the integrity of the stent graft and evaluate for fractures or migration. Minimal variability between the measurements for the minor axis of the aorta was noted between the two diagnostic modalities. Using the abdominal CT scan as the gold standard for post-EVAR evaluation, duplex ultrasonography was demonstrated to have a sensitivity of 42%, a specificity of 96%, a positive predictive value of 54%, and a negative predictive value of 94%. This has been corroborated by Arko and coworkers19 who examined post-EVAR follow-up CT and ultrasound studies in 201 patients. No significant difference was identified on paired analysis of maximal aortic diameter measurements with either imaging technique. Graft patency was documented to be 99% by both CT scan and duplex ultrasound. Furthermore, in comparison with CT imaging, duplex ultrasonography was shown to have a sensitivity of 81%, a specificity of 95%, a positive predictive value of 94%, and a negative predictive value of 90% for diagnosing an endoleak. The few endoleaks missed by duplex ultrasonography but diagnosed by a CT scan involved small lumbar arteries.
In addition to information regarding aneurysm sac size and endoleaks, iliac limb or outflow graft stenosis after EVAR can be easily diagnosed on duplex ultrasound.21 Access vessels such as the external iliac arteries are often atherosclerotic with heavy calcification and may need predilation before advancing large sheaths and placing stent grafts. Hemodynamically significant stenosis may require future treatment with balloon angioplasty or stenting. Access vessel complications during EVAR, including dissection, bleeding, false aneurysm, arterial thrombosis, and arterial embolization, were reported in 13% of the patients in the EUROSTAR registry.37 Subsequent iliac stent graft stenosis has been reported in 5.5% to 9% of cases.31 Therefore, the evaluation of iliac graft limbs and the native iliac arteries is an essential part of post-EVAR duplex ultrasound surveillance.
Migration of the stent graft, defined as greater than 10-mm displacement, has an incidence of 1.4% during the first year post-EVAR.31,38 Rarely, graft material may impinge upon the orifices of the renal arteries owing to either a short aneurysm neck or inaccurate deployment of the graft. A compromised renal artery can often be salvaged with stenting during the initial EVAR procedure or during a subsequent angiographic evaluation. It is important for duplex ultrasonography to evaluate the patency and any degree of stenosis in the renal arteries. Although not specifically validated in patients after EVAR, standard interpretation criteria for native renal arteries are usually applied. The duplex criteria for diagnosis of 60% or greater native renal artery stenosis include peak systolic velocity of 200 cm/s or greater and renal-to-aortic velocity ratio (RAR) of 3.5 or greater.39 There are no standard duplex criteria for stenosis in stented renal arteries, although experience suggests that velocity thresholds for significant stenosis in stented renal arteries are higher than those for native renal arteries (see Chapter 24).
Duplex ultrasound can be used for post-EVAR follow-up and detection of endoleaks with aneurysm sac enlargement. Figure 16.3 shows a patient with a type II endoleak followed up for 2 years. During the EVAR procedure, the patient had a complication with endograft migration requiring stenting of the left renal artery. It was noted on duplex ultrasound that despite a small persistent type II endoleak, the aneurysm sac had grown in diameter from 4.9 to 5.6 cm during a 15-month period. An arteriogram revealed an endoleak originating from the iliolumbar artery off the right internal iliac artery, which was then successfully coil embolized (Fig. 16.4).
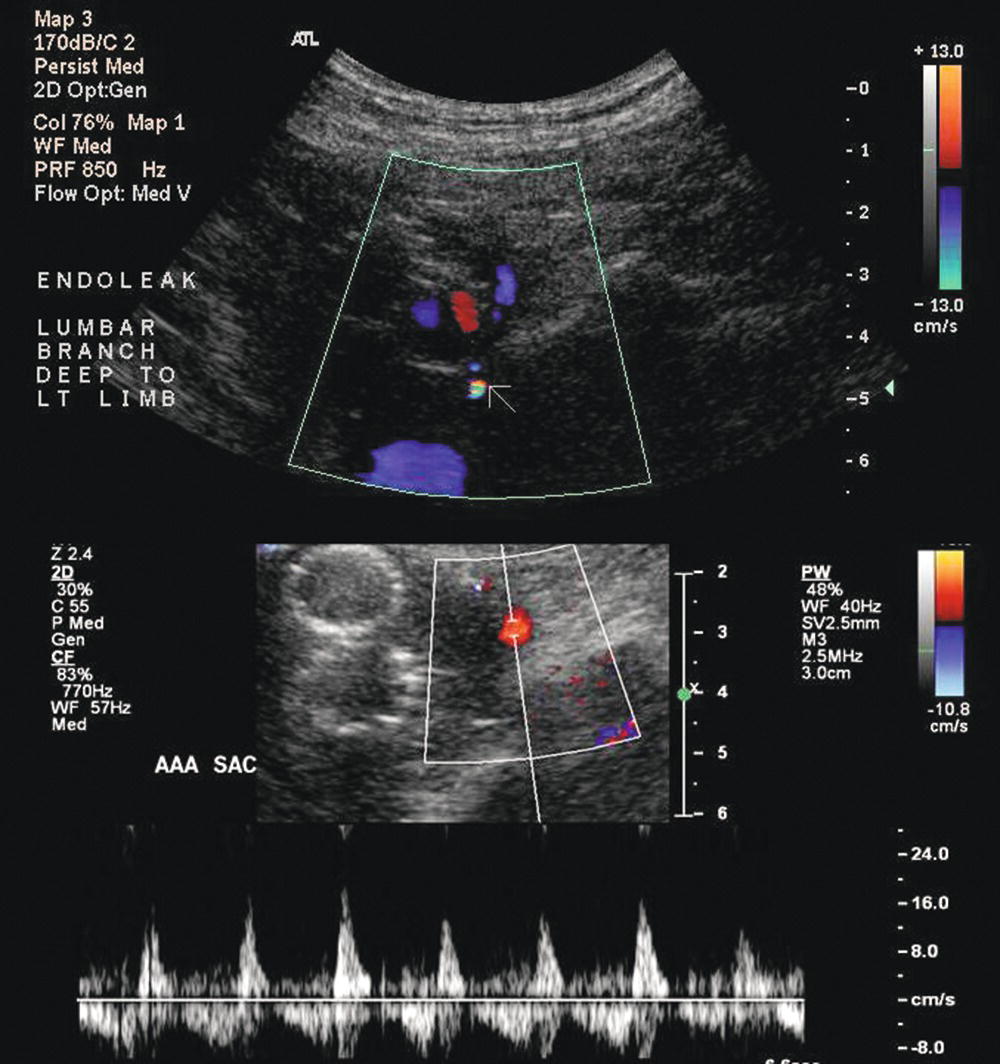
FIGURE 16.3. Duplex ultrasound images of a type II endoleak originating from a lumbar artery. Color flow image (top) shows an area of flow outside of the graft limbs (white arrow). Pulsed Doppler spectral waveform (bottom) shows a “to-and-fro” flow pattern with the sample volume positioned in the aortic aneurysm sac.
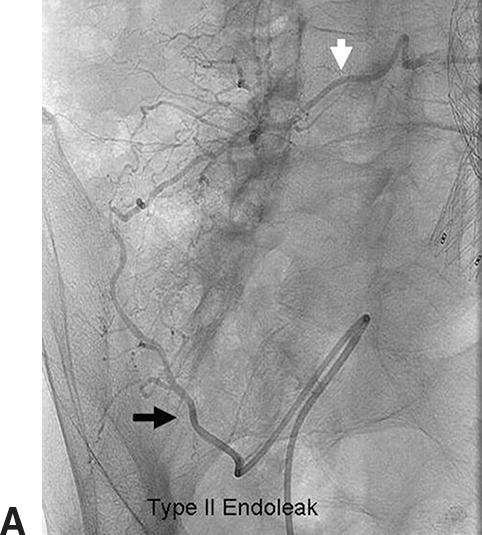
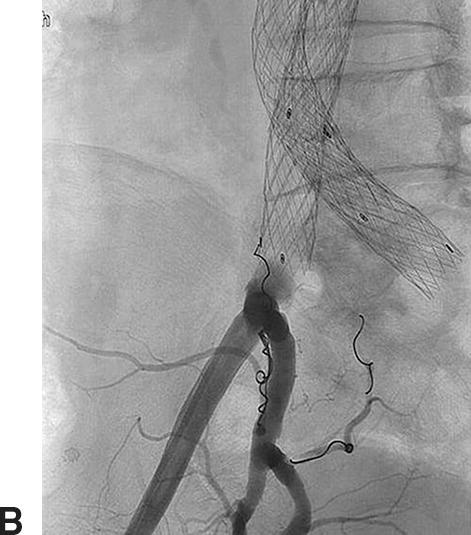
FIGURE 16.4. Type II endoleak. A,Angiogram confirms a right iliolumbar branch (black arrow) from the right internal iliac artery feeding a lumbar artery (white arrow). B,Coil embolization of the branch.
Aortic intrasac Doppler velocities and flow patterns may also help predict the natural history of endoleaks. Arko and coworkers19 found that a type II endoleak was more likely to seal spontaneously if it was associated with an intrasac Doppler flow velocity less than 100 cm/s, a small patent inferior mesenteric artery, or fewer paired lumbar arteries. Intrasac velocities less than 100 cm/s correlated well with the likelihood that an endoleak would thrombose without further treatment. Parent and associates29 observed that type II endoleaks with a “to-and-fro” flow pattern were more likely to resolve spontaneously, while those with a biphasic or monophasic flow pattern tended to persist. A more recent study by Beeman and coworkers15 concluded that intrasac flow velocities in type II endoleaks did not correlate with spontaneous resolution or increases in aneurysm sac size; however, the presence of multiple type II endoleaks and a bidirectional flow pattern were the strongest predictors of an increase in aneurysm sac diameter.
OVERVIEW OF POST-EVAR DUPLEX SCANNING
A general approach to duplex ultrasound for follow-up of aortic endografts can be based on aortic aneurysm sac measurement, stent device patency and integrity, presence of endoleaks, stenosis of outflow graft vessels, and any incidental findings such as renal artery stenosis or thrombus within the graft (Table 16.1). The basic components of the post-EVAR duplex ultrasound examination are aneurysm sac diameter measurement and evaluation for the presence of flow outside the stent graft to identify an endoleak. Determination of the type of endoleak is important, whenever possible, because it may determine the need and urgency for endoleak treatment. This requires fastidious scanning technique by the technologist and the use of multiple views. Measuring the flow velocity in the iliac limbs of the graft and immediately distal to the limbs can provide information about stenosis involving the distal stent graft attachment sites.
TABLE 16.1 Components of Post-EVAR Duplex Ultrasound

Although migration of the stent graft proximally is much less common than migration distally, the renal arteries should be evaluated by duplex ultrasound for any evidence of stenosis secondary to graft material impingement. The common femoral arteries should be evaluated to look for any evidence of access site complications, whether a cutdown or a percutaneous approach was used. Finally, measuring the ankle-brachial indices (ABIs) is important for assessment of arterial perfusion of the lower extremities, and the postoperative ABIs should be compared with preoperative values.
A variety of endografts for infrarenal AAA repair are currently approved for use in the United States (Fig. 16.5). All of these devices consist of graft material supported by self-expanding metal stents. The stent material may be nitinol, stainless steel, or cobalt-chromium alloy. The graft fabric material can be woven polyester or expanded polytetrafluoroethylene (ePTFE). The proximal fixation of the graft can be based on outward radial force of the stent material in the neck of the aneurysm, column strength of the device, or suprarenal stent components. With suprarenal fixation, the device has uncovered metal components above the fabric-covered infrarenal stent that help to secure the graft to the aortic wall above the renal arteries. Suprarenal fixation is intended to provide more secure fixation of the graft to the aortic wall, improve the seal, and reduce the risk of distal component migration. The use of fenestrated and branched endovascular stent grafts for complex aortic aneurysms has increased in recent years, and a commercially available fenestrated graft system for juxtarenal aneurysms is available in the United States.
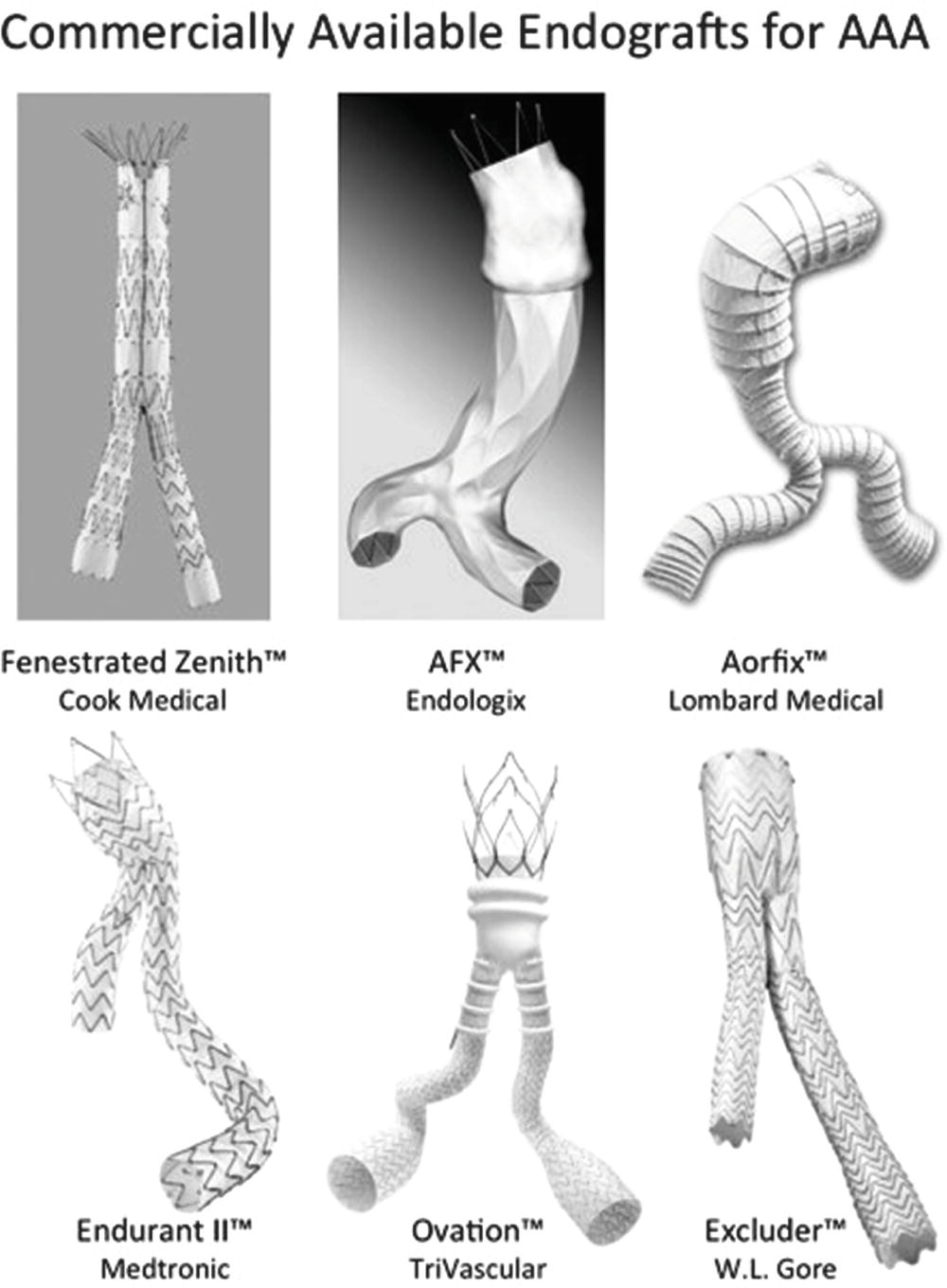
FIGURE 16.5. Six commercially available aortic endografts. Images courtesy of Cook Medical, Endologix, Lombard Medical, Medtronic Endovascular, TriVascular, and W.L. Gore and Associates.
Most aortic endografts are bifurcated modular systems with either one or two iliac limbs that “dock” with the aortic main body device. The two iliac limbs of the graft lie in the aortic aneurysm sac, sometimes crossing, and are easily identified on duplex ultrasound. A unibody bifurcated graft is also available that can be seated on the native aortic bifurcation. It is designed to reduce the risk of migration and the need for using multiple stent graft pieces. Newer devices have expanded the indications for EVAR, allowing endovascular treatment of aneurysms with short or angulated aortic necks, or with anatomically challenged access vessels (e.g., tortuous iliac arteries). An important consideration regarding the different stent graft devices available is that endoleak rates may differ based on the type of aortic endovascular graft device.12
Although endoleaks are relatively common, their significance and natural history are not completely understood, and many can be followed for months to years without intervention. A persistent endoleak many months postprocedure, or one associated with aneurysm sac enlargement, can be treated by a number of techniques, including transfemoral, transbrachial, or translumbar approaches. One frequently used technique consists of angiography for localization of the endoleak branch vessel and embolization with coils, glue, or other materials. Platinum and stainless steel are commonly used coil materials. Coil embolization may be achieved via access through an internal iliac artery branch connecting to a lumbar artery or through a branch of the superior mesenteric artery connecting to a branch of the inferior mesenteric artery. Open surgical or laparoscopic ligation of the vessel responsible for a type II endoleak is less frequently performed.
SCANNING TECHNIQUE
Instrumentation
Duplex evaluation of abdominal aortic endografts is one of the most technically challenging and complex examinations performed by vascular technologists. A full-featured ultrasound system with enhanced B-mode, harmonic imaging, high-quality color-flow imaging, high spectral Doppler sensitivity, and other imaging adjuncts better enable the technologist to perform a complete and accurate study. Transducer frequencies ranging from 2 to 5 MHz are used to evaluate the abdominal aorta and visceral vessels. Probe selection depends on the depth of the desired field of view. A low-frequency curved linear array is used to interrogate the abdominal aorta and iliac arteries from an anterior or lateral approach. A small-footprint low-frequency–phased array transducer can be used for intercostal views.
Historically, color flow Doppler imaging has been used as a guide and not as a primary diagnostic tool for arterial examinations. However, color flow Doppler is a requisite part of the abdominal aortic endograft evaluation. Color flow facilitates visualization of flow within the aortic aneurysm sac in the evaluation for endoleaks (see Fig. 16.3). The color flow Doppler pulse repetition frequency (PRF or color scale) should be set low to allow detection of slow flow velocities within the aneurysm sac. A small-sized color box will enable selective positioning within the aneurysm sac and maximize the frame rate. Persistence should be at a medium level. To correctly adjust color gain settings, the technologist should turn the color gain up to the point at which the color “noise” or color speckling is visible on the display and then reduce the color gain to the threshold at which the color “noise” is eliminated. This will ensure that the color gain is set at the highest possible setting without being “overgained.”
Patient Preparation and Positioning
Whenever possible, the patient should avoid gas-producing foods for several days before the study and avoid chewing gum or smoking before the examination to minimize intestinal activity and bowel gas. Ideally, the patient should also fast (if not medically contraindicated) for approximately 4 to 6 hours before the examination. Taking medications with a small amount of water is acceptable.
The examination starts with the patient supine and adequately exposed for imaging the abdomen, flanks, and groins. Abdominal aortic endograft evaluations are technically challenging and require numerous views and careful evaluation to detect subtle abnormalities. In general, about 1 to 2 hours should be scheduled for the examination to provide sufficient time to prepare the patient, perform the scan, and generate the preliminary report.
To avoid fatigue and reduce the risk of repetitive stress injury, arrange the imaging system, technologist chair, and patient scanning bed or stretcher for the comfort of both the patient and the technologist. The technologist should be positioned higher than the scanning surface, and the technologist’s arm should be supported and moderately flexed and positioned as close to the midline as possible. The technologist may stand during the examination to help apply probe pressure when necessary. If standing, the technologist should attempt to hold the scanning arm as straight and as close as possible, which will allow the technologist to “lean” into the scanning field and apply gentle pressure to displace mobile viscera and position the probe closer to the aorta. The availability of a low-profile step stool may be helpful. The technologist should make the patient aware that pressure may be applied with the ultrasound probe and instruct the patient to report any discomfort. The astute technologist should be aware of the patient’s comfort level at all times during the examination. Although probe pressure may be required for best imaging, the technologist can work with the patient to obtain optimal images with the least amount of discomfort.
It may be useful to have the patient turn to the side for right or left lateral decubitus scanning approaches. A lateral scanning approach can help overcome limitations due to depth, vessel tortuosity, and bowel gas. Varying approaches may also improve the patient’s comfort level by avoiding continuous probe pressure in the same region throughout the entire examination. It is important to remember that despite proper imaging techniques, image quality may be compromised by bowel gas, body habitus, abdominal wall hernias, presence of ascites, and patient intolerance.35 Post-EVAR examinations are advanced vascular laboratory studies. Therefore, it is important that the technologist learning to perform these studies be given sufficient time with the expectation that the number of technically limited examinations will decrease as experience is gained.
Examination Protocol
B-Mode Imaging
The examination begins with an anterior approach in a transverse imaging plane. The abdominal aorta should be identified starting at the level of the celiac artery. For difficult evaluations, color flow Doppler can help with branch vessel identification. The hyperechoic walls of most endografts are readily distinguished from the native aortic aneurysm wall (Fig. 16.6). In a transverse view, the endograft is visualized within the abdominal aorta and should be surrounded by thrombus within the aneurysm sac. Most modular bifurcated endograft configurations have the device bifurcation within the abdominal aorta, with two limbs traversing the distal aorta into the iliac arteries. Below the device bifurcation, this results in a “double-barreled” appearance within the abdominal aorta proximal to the native aortic bifurcation (Fig. 16.7). Unibody bifurcated endografts may be seated directly on the aortic bifurcation, and separate iliac limbs will not be visible in the aorta.
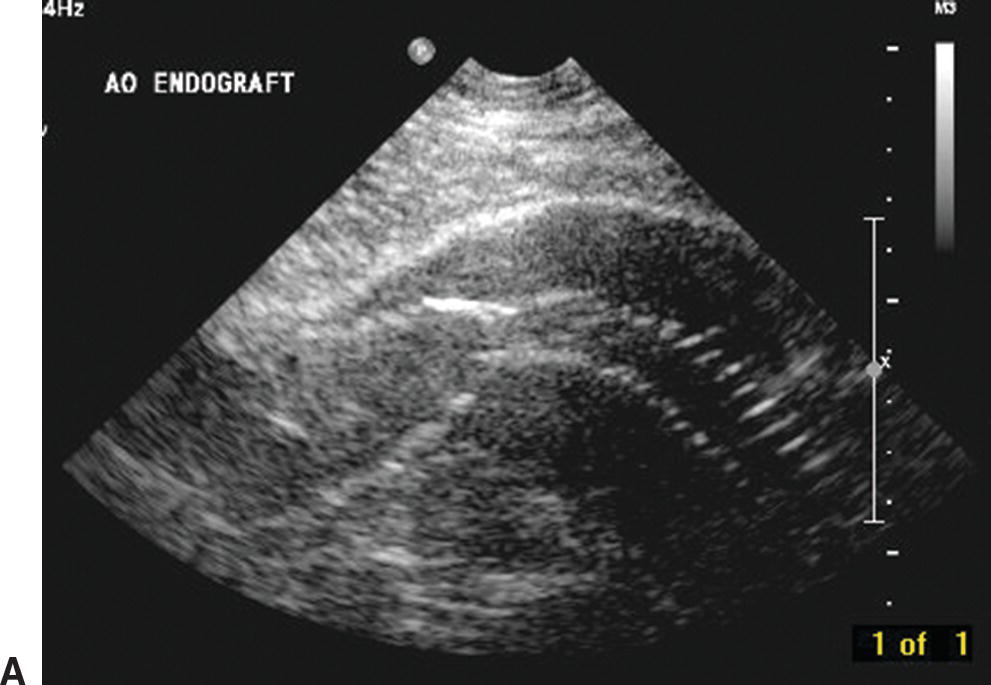

FIGURE 16.6. B-mode images of aortic endografts showing the hyperechoic graft walls within the aneurysm sac. A,Longitudinal view with the larger main body of the endograft on the left and a smaller iliac limb on the right. B,Transverse view of the main body of an endograft (inner ring) and the aneurysm sac (outer ring). Diameter measurements of the aneurysm sac are 4.47 cm × 4.93 cm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


