Dual-chamber Pacing: Special Considerations
Permanent Dual-Chamber Pacing
In Chapter 5, we discussed types of pacemakers and the hemodynamics of pacing, so now we can take a closer look at some of the features unique to dual-chamber pacing.
Lower Rate Limit of DDD Pacemakers
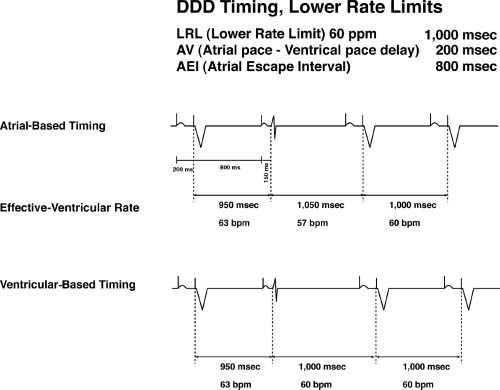
In the DDD pacemaker, even the simple lower rate limit paced interval becomes more complex. Basically two types of timing intervals are available: ventricular-based timing and atrial-based timing.
Ventricular-based timing is defined by timing intervals measured from the ventricular event (either a paced or a sensed ventricular beat). As shown in Figure 6-1, once the ventricular spike fires (or a native ventricular beat is sensed), the timing cycle starts, and then after the atrial escape interval, if nothing is sensed, the pacemaker will pace in the atrium. Again assuming no conduction through the AV node, the AV interval (the time from the atrial spike to the ventricular spike) completes a cycle. The heart rate is determined by the combination of the AEI and AVI.
Atrial-based timing is defined as the timer being started by an atrial event (either atrial pacing or sensing). A sensed atrial event such as a native P wave or a premature atrial beat will terminate and reset the AAI timer (Fig. 6-1). Assuming no native atrial activity occurs, the programmed atrial escape interval will occur and an atrial spike will fire in the atrium, and assuming no conduction through the AV node, ventricular spike will occur. Once again the lower rate limit, in this situation, is determined by the AEI and AVI together. In the examples above, in each case the basic rate is 60 bpm with a total AEI and AVI of 1,000 msec.
At first, these timing cycle differences may seem academic and, in fact, the differences are often relatively minor. On the other hand, they can lead to confusion. For instance, with ventricular-based timing, a ventricular event could occur and start the timer. A paced atrial event could then occur, but with conduction through the AV node at a shorter interval than the programmed AVI. Thus the total AEI and AVI in this situation would be less than
1,000 msec. and the intrinsic lower rate limit would actually be greater than 60 bpm even though the pacemaker is functioning appropriately.
1,000 msec. and the intrinsic lower rate limit would actually be greater than 60 bpm even though the pacemaker is functioning appropriately.
With atrial-based timing, the patient could be pacing in the atrium and ventricle, and then a PVC could occur (Fig. 6-2). Generally that would allow resetting of the timer, but an entire AA interval would, in early pacemakers, result in a very prolonged interval after the PVC (even longer than the compensatory
pause that occurs naturally in the absence of a pacemaker). Ventricular bigeminy can lead to a worrisomely slow rhythm in that setting.
pause that occurs naturally in the absence of a pacemaker). Ventricular bigeminy can lead to a worrisomely slow rhythm in that setting.
A commonly used type of timing is modified atrial-based timing (refer to Fig. 6-2). This is atrial-based timing, but modified to shorten ventricular pacing after a PVC. As shown in Figure 6-2, after a PVC rather than time out an entire AA interval (for example, 1,000 msec), it will shorten that post-PVC interval by the programmed AV delay (for example, 200 msec). Modified atrial-based timing shortens the compensatory pause after a PVC in the hope of leading to a less symptomatic event.
Cross-Talk and Ventricular Safety Pacing
In a dual-chamber pacing system with atrial and ventricular pacing and ventricular sensing, the atrial pacemaker spike or its after-potential could be sensed by the ventricular system and inhibit ventricular firing (the ventricular system may interpret the atrial event as a ventricular contraction). This occurrence is referred to as cross-talk and pacemakers are designed to avoid cross-talk using various methods. Cross-talk is more likely to occur if the atrial lead is unipolar (because of the large spike and after-potential) than if it is bipolar.
We mention cross-talk and blanking periods in Chapter 5. Basically the ventricular lead system does not sense the atrial sensing spike because of the blanking period which is automatically triggered by the atrial spike.
The likelihood of cross-talk is increased if the ventricular lead is placed in the right ventricular outflow tract (physically closer to the atrium), if the atrial lead is physically located in the low atrial septum, if the atrial spike voltage is programmed high, or if the ventricular sensitivity is programmed high (i.e., more sensitive).
In a patient with complete AV block, it could be disastrous to the patient if pacing spikes or P waves were sensed as QRS complexes and ventricular pacing was totally inhibited. Algorithms are put in place to protect the patient from this occurring. During the first 120 msec of the AV interval, there is first a brief blanking period of a few milliseconds. This is followed by a noise sampling window and if any signal, physiologic or otherwise, is detected during that time, that signal will trigger ventricular pacing at the completion of the noise sampling window or shortly thereafter. Thus, if a P wave is sensed, there will still be ventricular pacing. This does carry the potential minor disadvantage of a true QRS complex having occurred there and the pacemaker pacing on top of that, but this is not at an interval that is likely to cause a pacemaker spike to land on the T wave of that QRS and so is of no real risk to the patient.
Pacemaker-Mediated Tachycardia
Figure 6-3 demonstrates a pacemaker-mediated arrhythmia that has been referred to as an endless loop. In either a VDD or DDD pacemaker, sensing occurs in the atrium and triggers the ventricle. Retrograde AV (ventricle-to-atrium [VA])
conduction occurs in response to ventricular pacing, which causes atrial contraction, shown schematically as an inverted P wave earlier in Figure 6-1 (do not expect the P wave to be so readily visible in clinical practice). Sensing of the P wave causes the ventricle to be stimulated, completing the endless loop or circus tachycardia.
conduction occurs in response to ventricular pacing, which causes atrial contraction, shown schematically as an inverted P wave earlier in Figure 6-1 (do not expect the P wave to be so readily visible in clinical practice). Sensing of the P wave causes the ventricle to be stimulated, completing the endless loop or circus tachycardia.
Figure 6-3 shows the problem of slow retrograde AV conduction. If the VA conduction occurred rapidly, it would tend to fall into the postventricular atrial refractory period (PVARP), during which atrial sensing is ignored, and no endless loop arrhythmias would occur. The length of the PVARP is programmable in modern DDD pacemakers. It extends from the ventricular event (paced or sensed QRS) to whatever length it is programmed (usually 250 to 275 msec).
Not all patients have VA conduction, but even some patients with complete antegrade AV block may have intact retrograde AV conduction. Electrophysiologic testing for VA conduction before implantation of a DDD or VDD unit is useful, but the VA conduction may be intermittent.
The tools to treat pacemaker-mediated tachycardia (PMT) are basically to extend PVARP and the presence of certain algorithms to terminate PMT.
Initially attempts were made to block VA conduction with medications. These were usually unsuccessful and, in fact, could aggravate the problem by delaying VA conduction to beyond the programmed PVARP.
Basically if a retrograde P wave is occurring and being sensed, it will not be responded to by extending the PVARP. It is of note that retrograde P waves may be triggered by a premature ventricular contraction (PVC). Some pacemakers generally have an automatic extension of PVARP after a PVC to combat this potential source of PMT.
Pacemakers also have algorithms to interrupt suspected PMT. It will not prevent PMT, but they are designed to terminate PMT if it occurs. For example, a pacemaker can be programmed not to sense the sixteenth atrial beat if a pacemaker unit is running at or near its upper rate limit, thus interrupting PMT, but not causing problems if the rapid rate is caused by sinus tachycardia or some other form of supraventricular tachycardia (SVT). The algorithms can be complex. For example, intermittent extension of PVARP, disabling the sensing amplifier briefly, or trying to determine whether the PMT is present by VA timing measurements are all potential algorithm devices that can be used to terminate PMT.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree