Diving Injuries and Air Embolism
During the winter of 1942–1943, Jacques Cousteau and Emile Gagnan collaborated to develop and patent the first commercially successful open-circuit self-contained underwater breathing apparatus (SCUBA), which was later named the Aqua Lung. The apparatus consisted of a demand valve regulator that could be attached to a portable cylinder containing stored compressed breathing gas. The regulator reduced the relatively high pressure of the stored gas to ambient pressure, which could then be breathed by a diver submersed in water. Prior to the development of reliable SCUBA equipment, divers were limited by surface supplied breathing gas requiring direct and continuous surface support.
The mass production of SCUBA diving equipment revolutionized underwater exploration. While this was a significant development for the military and commercial diving communities, it marks the beginning of the sport diving era. SCUBA diving has since evolved into a popular recreational activity.
Although diving-related injuries were described prior to the development of modern-day SCUBA equipment, their relative importance increased as more divers became equipped to explore the underwater world. The most accurate available statistics of diving-related injuries are published online by the Divers Alert Network (DAN) in an Annual Review of Recreational Scuba Diving Injuries and Fatalities.
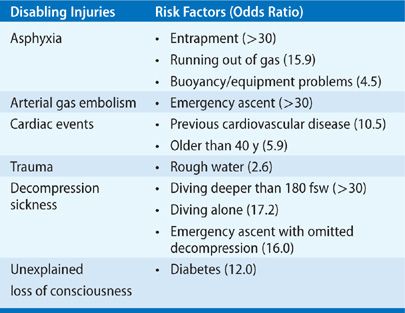
A recent detailed and thorough analysis of 947 recreational SCUBA diving fatalities from 1992 to 2003 identified the cause of death (COD) along with triggers, disabling agents, and disabling injuries that contributed to the COD.1 The leading COD was drowning (70%), but the identifiable disabling injuries that contributed to the COD told a more useful story. The most common disabling injuries included: asphyxia with no preceding disabling injury (33%), arterial gas embolism (AGE) (29%), cardiac events (26%), trauma (5%), decompression sickness (DCS) (3%), unexplained loss of consciousness (2%), and inappropriate gas mixture (2%). In addition, risk factors associated with each disabling injury were identified (Table 93-1).
TABLE 93-1 Disabling Injuries and Associated Risk Factors Contributing to the Cause of Death in Recreational Diving Fatalities
Of all the possible causes of diving-related injuries, this chapter focuses only on those caused by dissolved or embolic gas. In addition, this chapter includes a discussion of nondiving-related gas embolism. A number of excellent sources for review of diving-related injuries are available.2–7
FUNDAMENTAL CONCEPTS
Before presenting individual clinical entities, fundamental concepts related to pressure and the behavior of gases are reviewed.
 PRESSURE
PRESSURE
Pressure is defined as force per unit area. The air-containing atmosphere of the earth applies pressure to any object within it. At sea level this pressure is defined as one atmosphere (1 ATM). One atmosphere is equivalent to 760 torr (mm Hg), 29.92 in Hg, 14.7 psi, 101.3 kPa, or 1.013 bar. Most of the world’s population lives at or near sea level and rarely experiences significant changes in ambient pressure. Divers are unique in that they experience significant increases in ambient pressure as they descend through the water column. Every 33 ft of sea water (fsw) through which a diver descends adds an additional 1 ATM of pressure. By the time a diver reaches a depth of 99 fsw, he or she has added an additional 3 ATM to the earth’s atmospheric pressure. The diver experiences a pressure of 4 atmospheres absolute (4 ATA) at this depth. The pressure subsequently decreases as the diver ascends back to the surface.
 BOYLE’S LAW
BOYLE’S LAW
Originally described by Sir Robert Boyle in 1662, Boyle’s Law describes the relationship between pressure and volume of a fixed amount of an ideal gas given that the temperature of the system remains constant (Video 93-1), stating that pressure and volume are inversely proportional. It is important to note that because the relationship is exponential, larger changes in volume occur with changes in pressure over a range of lower pressure values.
Video 93-1 This animation demonstrates the inverse relationship between pressure and volume of an ideal gas given a fixed mass and constant temperature. Note from the graph that the relationship is exponential. Larger changes in volume are noted with pressure changes at the smaller pressure values. Also, note that the density of the gas increases with increased pressure. Access at www.fishmansonline.com
 BAROTRAUMA
BAROTRAUMA
Barotrauma is physical damage to tissues within the body that occurs as a result of changing ambient pressure. Tissues that contain air spaces are at risk. Barotrauma can be considered a physiological ramification of Boyle’s Law.
Middle ear barotrauma (MEB) is the most common diving-related injury. As a diver descends in the water column he or she must be able to equalize the pressure within the air-containing middle ear space to the surrounding increasing ambient pressure. If the pressure cannot be equalized by adding gas to the space during descent, the volume within the middle ear space will contract according to Boyle’s Law. Proper eustachian tube function is critically important in the prevention of MEB; divers can utilize different maneuvers to assist in the equalization of middle ear pressures.
The most common manifestation of MEB is ear pain experienced during descent. Other manifestations include changes in the appearance of the tympanic membrane with possible rupture, the evolution of a middle ear effusion (with or without blood), decreased hearing, and mild vertigo. Injuries associated with MEB are usually transient and reversible. Treatment is supportive and may include the use of decongestants, along with avoiding further changes in ambient pressure.
Other less frequent forms of barotrauma include situations where gas becomes trapped in the sinuses, gastrointestinal tract, and within a tooth filling. Pulmonary barotrauma is discussed later in the chapter.
 DALTON’S LAW OF PARTIAL PRESSURES
DALTON’S LAW OF PARTIAL PRESSURES
Originally described by John Dalton in 1801, Dalton’s Law states that the total pressure exerted by a gas mixture is equal to the sum of the partial pressures of each individual gas component.
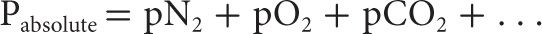
The partial pressure of a gas component within the mixture can be determined by multiplying the fraction of the component gas by the absolute pressure. For example:
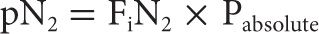
It is important to note that as absolute pressure increases, so does the partial pressure of each individual gas component within the gas mixture. A SCUBA diver breathing air (78% N2, 21% O2) descending to a depth of 99 fsw (4 ATA) is exposed to elevated partial pressures of each of the component gases within the mixture. The partial pressure of nitrogen in the inspired air at this depth is significantly increased (~3.1 ATM).
There are physiological ramifications of exposure to elevated partial pressures of specific gases. Exposure to elevated partial pressures of nitrogen causes nitrogen narcosis and provides a pressure gradient, driving dissolved nitrogen into tissues of the body. Exposure to elevated partial pressures of oxygen causes oxygen toxicity, which can manifest as seizure activity and loss of consciousness without warning. This becomes a significant concern for divers breathing mixtures with increased FiO2 or those who dive to extreme depth.
 HENRY’S LAW
HENRY’S LAW
Originally described by William Henry in 1803, Henry’s Law states that the amount of a given gas that dissolves in a liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid given at constant temperature. An alternative explanation is that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid.
An air-breathing diver descending in the water column experiences a pressure gradient which drives dissolved nitrogen into the tissues based on Dalton’s Law; in addition, the solubility of nitrogen within the tissues also increases according to Henry’s Law. During decompression the amount of nitrogen that can remain dissolved decreases, favoring transition to gas phase and the evolution of gas bubbles.
An understanding of both Dalton’s Law and Henry’s Law is important because these laws contribute to inert gas (i.e., nitrogen) exchange. The tissues of a diver will load or “on-gas” inert gas during compression and with time at depth. Unloading or “off-gassing” occurs during decompression and with time once the diver has reached the surface again.
Physiological ramifications of Henry’s Law include DCS, which is discussed later in the chapter.
DECOMPRESSION ILLNESS
The term decompression illness (DCI) refers to disease processes caused by gas bubbles that result from a reduction in ambient pressure. The term includes both dysbaric AGE and DCS.
 PULMONARY BAROTRAUMA
PULMONARY BAROTRAUMA
If a diver were to descend while holding his or her breath, the gas within the lungs would be compressed progressively while maintaining a volume that is inversely proportional to the increasing pressure. In order to prevent collapse of the lung to less than residual volume, with tearing of pulmonary parenchyma and blood vessels, the diver is obliged to breathe an oxygen-containing gas mixture at a pressure equal to that of the surrounding water. During return to normal atmospheric pressure, compressed gas within the lungs expands exponentially and must be exhaled if alveolar rupture is to be avoided.
The greatest danger of alveolar bursting occurs during ascent in the shallowest depth range because of the exponential inverse relationship between pressure and volume according to Boyle’s Law. Fatal AGE associated with breathing compressed gas has been reported following ascent from a depth of only 4 fsw.8
 POSSIBLE SEQUELAE OF ALVEOLAR RUPTURE DURING DECOMPRESSION
POSSIBLE SEQUELAE OF ALVEOLAR RUPTURE DURING DECOMPRESSION
The sequelae of pulmonary overpressure accidents are determined by the nature and severity of associated tissue trauma as well as by the volume of expanding extra-alveolar gas. Following rupture of alveolar septa, expanding gas enters the interstitial spaces and dissects along perivascular sheaths to enter the mediastinum. Mediastinal gas may further dissect into the pericardial sac, the retroperitoneal space, or the subcutaneous tissues of the neck. Gas also may enter the pleural space to cause a pneumothorax.
Mediastinal emphysema is often associated with mild substernal discomfort that may be described as a dull ache or a feeling of tightness. Deep inspiration, coughing, or swallowing may exacerbate symptoms, and pain may radiate to the shoulders, neck, or back. Unless extensive, mediastinal emphysema is rarely associated with dyspnea, tachypnea, or other signs of respiratory distress. Gas volumes within the pericardial sac or retroperitoneal space are seldom large enough to be clinically significant.
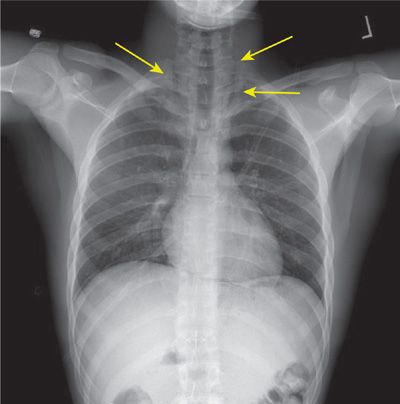
Subcutaneous emphysema from pulmonary barotrauma causes swelling and crepitance in the neck and supraclavicular fossae. These signs may be associated with sore throat, dysphagia, or a change in voice tone. Subcutaneous gas can be demonstrated radiographically (Fig. 93-1). If symptoms of mediastinal or subcutaneous emphysema are bothersome, resolution of gas can be hastened by breathing 100% oxygen at normal atmospheric pressure. Recompression therapy is not recommended as it may exacerbate the underlying lung injury and add compressed gas to the spaces followed by expansion during decompression from treatment.
Figure 93-1 Subcutaneous emphysema (arrows) secondary to pulmonary barotrauma is demonstrated radiographically. This novice diver, participating in his initial training dives, was attempting to equalize his middle ear pressures using a forced valsalva maneuver during ascent.
Surprisingly, pneumothorax is not a frequent complication of diving-related pulmonary barotrauma. In one series of submarine escape ascents, pneumothorax occurred in only 5% to 10% of the divers who had lung overinflation syndromes and suffered cerebral AGE.9 Recompression of an individual who is known to have a pneumothorax should be avoided if possible. However, it may be necessary if neurological symptoms or other manifestations of AGE are present. Insertion of a thoracostomy tube prior to recompression treatment is recommended.
Conversion from an untreated simple pneumothorax to a tension pneumothorax can occur during recompression therapy in a hyperbaric chamber. This will occur if a tear in the visceral pleura remains open during compression, thereby allowing compressed gas to enter the pleural space. If the air in the pleural space becomes effectively sealed prior to or during decompression, the gas will expand to compress the lung and interfere with venous return. Severe dyspnea, cyanosis, and hypotension may occur. This is an emergency that requires immediate recompression to relieve symptoms and insertion of a chest tube before decompression is resumed. Alternatively, a pneumothorax can be managed by needle decompression and the use of a flutter (one-way) valve.
 DYSBARIC ARTERIAL GAS EMBOLISM
DYSBARIC ARTERIAL GAS EMBOLISM
When expanding extra-alveolar gas is forced down a pressure gradient into torn septal vessels, it traverses the pulmonary veins to the left atrium and left ventricle, from which it is ejected into the systemic circulation as foamy particles or discrete bubbles. Distribution of the gas emboli is determined by their buoyancy relative to blood and orientation of the body with respect to gravity. It may also be influenced by local factors such as blood flow and vessel size. With the body in the head-up, erect position, most of the embolic gas travels to the brain.
Although the precipitating event for dysbaric AGE is presumed to be due to pulmonary overinflation injury, demonstrable radiographic evidence of pulmonary barotrauma is not always seen. In one series of divers suffering symptomatic AGE, radiographic evidence of pulmonary barotrauma was demonstrated in only 42% of cases.10
Clinical manifestations of dysbaric AGE have been grouped into two categories based on the initial presentation and response to treatment. About 5% of the divers who experience AGE are critically injured and often die even when recompression is initiated within minutes.11 These individuals develop apnea, unconsciousness, and cardiac arrest during ascent or immediately after surfacing from a dive. The most likely cause of this frequently lethal condition is massive volumes of gas in the central circulation.
The majority of patients with dysbaric AGE present with neurological signs and symptoms, but spontaneous respiration and heart rate are maintained. Just as in the more seriously injured divers, onset of symptoms occurs during ascent or within minutes after surfacing. The clinical spectrum of neurological disturbances ranges from focal signs such as monoparesis or discrete sensory deficits to diffuse brain dysfunction, as manifested by confusion, stupor, or coma. In response to prompt recompression and hyperbaric oxygenation, most patients have complete resolution of all neurological deficits.
For reasons that are not well understood, a subgroup of these patients will fail to respond completely or will experience initial improvement followed by recurrence of the presenting signs and symptoms secondary to delayed cerebral edema.12 The probability of incomplete response or recurrence is increased as the time between onset of symptoms and initiation of definitive therapy is prolonged.
The true incidence of cerebral AGE may be higher than that established on the basis of historical and physical findings alone, as electroencephalographic evidence of abnormal neuronal activity has been demonstrated after submarine escape training ascents in the absence of associated clinical manifestations.13
 IATROGENIC AND ACCIDENTAL ARTERIAL GAS EMBOLISM
IATROGENIC AND ACCIDENTAL ARTERIAL GAS EMBOLISM
Accidental AGE is a serious and sometimes lethal complication of many procedures that are widely used in modern medicine.14 It is often misdiagnosed or recognized only after a delay of several hours. Most cases of AGE present with focal or diffuse manifestations of brain ischemia. Management is often made more difficult by the existence of concurrent medical or surgical complications. In many patients, hyperbaric oxygen therapy, if administered promptly, completely reverses all neurological deficits. It is generally remarkably efficacious even when initiated after a delay of several hours. For a list of surgical procedures that have been associated with iatrogenic AGE, see Table 93-2.