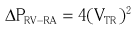Chapter 24 In the setting of chronic tricuspid or pulmonic regurgitation, the right ventricle dilates in response to chronic volume overload, which is visualized on echocardiography predominantly as enlargement in the short axis rather than in the longitudinal axis dimension.1,2 Right ventricular volume overload is also associated with abnormal or “paradoxic” ventricular septal motion, because the septum moves toward the center of the right ventricle in systole and moves rapidly posteriorly in diastole—a pattern opposite of normal.3–5 The reversed curvature of the septum is most marked in end-diastole, in contrast to pressure overload in which the maximum reversed curvature is more evident and occurs early in diastole. 6 In chronic right ventricular volume overload, right ventricular systolic dysfunction occurs earlier in the disease course than is typical for left-sided volume overload conditions.7,8 As with left-sided valve disease, right ventricular volume and systolic function typically improve after intervention for valvular regurgitation unless an irreversible decline in contractility has occurred. The response of the right ventricle to chronic pressure overload, such as pulmonary hypertension or pulmonic stenosis, also differs from that of the left ventricle. Although the initial response is an increase in wall thickness, ventricular dilation may occur and depends on the acuteness and severity of the pressure overload state. With a gradual increase in right ventricular pressure, right ventricular size and systolic function may remain normal with a compensatory increase in right ventricular wall thickness. 9 After intervention, such as relief of pulmonic stenosis, an improvement in right ventricular hypertrophy and systolic function is expected because of the decreased right ventricular afterload. Although few studies have analyzed the extent of improvement in right ventricular function after relief of pulmonic stenosis, the improvement in right ventricular dimensions and systolic function in most patients after lung transplantation supports the concept that systolic function improves with decreased afterload.10,11 In contrast, with an acute increase in right ventricular pressure, for example with acute pulmonary embolism, decreased right ventricular systolic function and clinical right heart failure may be seen with mean pulmonary pressures of only 20 to 40 mm Hg. 12 Acute or subacute right ventricular pressure overload often results in right ventricular dilation with secondary annular dilation and tricuspid regurgitation. This combination superimposes a volume overload state, engendering a vicious circle of right ventricular dilation and worsening tricuspid regurgitation. After a thorough history and physical examination, echocardiography remains the cornerstone of diagnosis of right-sided valve disease and follows the same principles as those of evaluation for left-sided disease (see Chapter 6), confirming the presence and severity of valvular stenosis and regurgitation and providing important information about etiology. Assessment of the consequences of the valve abnormality for right ventricular size and function is also important. Although echocardiography can provide morphologic assessment of right ventricular dimensions and function, accurate measurements are difficult because of the complex three-dimensional (3D) anatomy of the right ventricle. 13 Three-dimensional echocardiographic imaging improves estimation of right ventricular volumes over two-dimensional (2D) imaging, but cardiac magnetic resonance (CMR) imaging is more accurate.14–16 For routine clinical assessment, tricuspid annular plane systolic excursion is a simple and reproducible measurement of right ventricular longitudinal function that correlates well with right ventricular ejection fraction by CMR.14,15 Other measures of right ventricular function, including the right-sided index of myocardial performance (Tei index) 17, measurements of the peak systolic velocity, and displacement of the tricuspid annulus using tissue Doppler imaging18–20 are feasible and have prognostic value in patients with pulmonary hypertension and other pathologies. 14 Right ventricular strain is a promising method of evaluating regional right ventricular contractility, and reduced strain predicts disease progression in pulmonary arterial hypertension.14,21 The extent of right ventricular hypertrophy can be assessed qualitatively from the thickness of the right ventricular free wall.13,14 The timing of ventricular septal motion also provides insight into right ventricular function. Although patterns of abnormal septal motion may be appreciated on 2D imaging, the timing and extent of septal motion are best evaluated using M-mode echocardiography. When right ventricular enlargement is present, careful assessment of the atrial septum and pulmonary veins is critical to exclude left-to-right shunt, and transesophageal echocardiography should be performed if uncertainty remains after transthoracic imaging. CMR methods for the assessment of right ventricular size and function are discussed in greater detail in Chapter 8. The normal tricuspid valve is characterized by three sail-like leaflets: anterior, posterior, and septal ( Figure 24-1). The anterior leaflet is the most anatomically constant of the three, the other leaflets varying more often in size and position. The leaflets are attached to the tricuspid valve annulus and are restrained by chordae tendineae attached to the papillary muscles, which in turn insert into the right ventricular wall. However, tricuspid valve chordae may also insert directly into the right ventricular free wall, a feature distinguishing the right and left ventricles. FIGURE 24-1 Tricuspid Valve Anatomy. Tricuspid regurgitation that is at least moderate or greater in severity is most frequently “functional” or secondary in nature. Secondary tricuspid regurgitation by definition is not due to primary tricuspid leaflet pathology but is secondary to another disease process causing right ventricular dilation, distortion of the subvalvular apparatus, tricuspid annular dilation, or a combination of these problems. Causes of clinically significant tricuspid regurgitation are listed in Table 24-1. Furthermore, a moderate or greater degree of tricuspid regurgitation, regardless of etiology, usually engenders worsening tricuspid regurgitation owing to adverse hemodynamic consequences of right ventricular volume overload, resulting in a slow and inexorable clinical and hemodynamic deterioration. TABLE 24-1 Causes of Tricuspid Valve Regurgitation Congenital Tricuspid Valve Disease Right Ventricular Disease Acquired Tricuspid Valve Disease Left-sided valvular heart disease Iatrogenic (irradiation, drugs, biopsy, pacemaker, implantable cardioverter-defibrillator) Right Ventricular Dilation Tricuspid regurgitation secondary to pulmonary hypertension is seen in patients with significant left-sided heart disease, those with primary pulmonary hypertension, and those with pulmonary disease leading to cor pulmonale. 22 As a general rule, when systolic pulmonary artery pressures increase beyond 55 mm Hg, tricuspid regurgitation can occur despite anatomically normal tricuspid leaflets, whereas more than mild tricuspid regurgitation occurring in the setting of lower systolic pulmonary pressures (<55 mm Hg) likely reflects a structural abnormality of the valve leaflets or the subvalvular apparatus.23,24 Secondary tricuspid regurgitation also results from tricuspid annular dilation in patients with right ventricular enlargement resulting from right ventricular infarction, dilated cardiomyopathy, or chronic left-to-right shunt due to an atrial septal defect or anomalous pulmonary venous drainage.25–27 Primary tricuspid valve pathology leading to tricuspid regurgitation may result from blunt trauma, iatrogenic injury, or specific diseases. When it is caused by permanent pacemaker or internal cardiac defibrillator leads, the mechanism of valve injury is variable, related to lead entrapment in the tricuspid apparatus, direct leaflet perforation, fibrotic adhesion of the lead to the leaflet, or avulsion or laceration of the tricuspid valve leaflets upon lead removal. 28 Because leaflet injury may be underappreciated, a high clinical index of suspicion is warranted, particularly when the patient with such an injury later presents with worsening right heart failure. Echocardiography, including 3D imaging, may be useful in localizing the leads relative to the tricuspid valve leaflets ( Figure 24-2). The device leads can be visualized on computed tomography, 29 but because of artifact from the leads, their position relative to the tricuspid valve leaflets can be difficult to determine. Tricuspid valve repair or replacement may be required in symptomatic patients; 28 the role of pacemaker or defibrillator extraction to improve tricuspid regurgitation in patients without infection is less clear. FIGURE 24-2 Device Lead Related Tricuspid Regurgitation. Direct tricuspid valve leaflet or chordal trauma may occur from transvenous endomyocardial biopsy, particularly in patients who have undergone cardiac transplantation who have repeated biopsies for rejection surveillance 30 ( Figure 24-3). Echocardiographic guidance using real-time three-dimensional imaging during the biopsy may prevent damage to the tricuspid valve or subvalvular apparatus. 31 FIGURE 24-3 Flail tricuspid valve leaflet that occurred as a complication of an endomyocardial biopsy. The tricuspid leaflets and supporting structures may be damaged by blunt chest trauma, most often after a motor vehicle accident resulting in papillary muscle, valve, or chordal rupture. Affected patients may be asymptomatic and remain so for years following the trauma, and the murmur of tricuspid regurgitation is often not initially recognized. 32 Conduction abnormalities, including right and left bundle branch block and left anterior hemiblock, occur in more than 90% of patients with traumatic tricuspid regurgitation. Severe tricuspid regurgitation due to a flail leaflet is associated with adverse outcomes favoring early surgical repair (see natural history discussion). 30 Damage to the tricuspid valve may also occur as a result of infective and marantic endocarditis.30,33 Right-sided infective endocarditis is usually a manifestation of intravenous drug abuse, indwelling dialysis or chemotherapy venous catheters, or infected pacemakers or implantable cardioverter defibrillators24,34–38 ( Figure 24-4). Staphylococcus aureus accounts for 80% of these tricuspid valve infections, although in pacemaker- or defibrillator-associated endocarditis, coagulase-negative staphylococcus may be more common.24,35,36 In cases of implantable cardiac device or indwelling catheter infections, early device extraction reduces mortality, and in most cases, the pacemaker or defibrillator can be explanted safely even with large vegetations.36,39 Infrequently, marantic or noninfective endocarditis occurs in the setting of systemic lupus erythematosus, rheumatoid arthritis, or antiphospholipid antibody syndrome. 40 The tricuspid valve may also be affected in up to 30% to 50% of patients with rheumatic valve disease. 41 FIGURE 24-4 Endocarditis associated with a device lead. Serotonin-active drugs can induce fibroproliferative changes to the tricuspid valve leaflets, which are mediated by the 5-HT2B receptor. 42 These changes result in pathologic and echocardiographic features similar to those seen in carcinoid heart disease, with thickened tethered leaflets leading to tricuspid regurgitation. This association was first described with the ergot alkaloids, ergotamine and methysergide, used for migraine therapy. 43 The anorectic agents fenfluramine and dexfenfluramine were subsequently implicated and have since been withdrawn from the market. 44 Pergolide and cabergoline, dopamine agonists used in the treatment of Parkinson disease and restless leg syndrome, induce valve thickening and regurgitation by a similar mechanism and have also been withdrawn.45–47 Carcinoid heart disease is a rare but distinctive form of valve disease affecting primarily the right-sided cardiac valves. Carcinoid tumors arise from argentaffin cells; the primary tumor is usually located in the small bowel and metastasizes to the liver. Serotonin produced by the primary tumor and metastases is recognized to be an agent involved in the development and progression of valve disease in patients with carcinoid syndrome. 48 Carcinoid heart disease involves a combination of tricuspid regurgitation ( Figure 24-5) with rare stenosis as well as pulmonic stenosis and regurgitation. Left-sided valvular involvement occurs in approximately 10% of patients with carcinoid, generally in relation to right-to-left shunting of serotonin-rich blood through a patent foramen ovale or primary lung metastases. 49 Rarely, carcinoid valve disease occurs in patients without hepatic metastases; an ovarian carcinoid tumor should be sought in this setting. 50 FIGURE 24-5 Tricuspid Regurgitation due to Carcinoid Heart Disease. Mediastinal irradiation can directly damage the tricuspid leaflets ( Figure 24-6). The associated post-inflammatory fibrosis and calcification, which usually manifest 5 years or longer after the radiation insult, result in distortion of the leaflets, causing tricuspid regurgitation.24,51,52 Assessment and treatment of tricuspid regurgitation in this setting may be complicated by concomitant dysfunction of other cardiac valves as well as pericardial, myocardial, and coronary artery involvement. FIGURE 24-6 Radiation-induced valvular heart disease in a patient treated for lymphoma. Congenital causes of tricuspid regurgitation are rare and include congenital tricuspid valve prolapse, which may occur as an isolated abnormality or may be associated with mitral valve prolapse and other connective tissue disorders.53,54 The most common congenital cause of tricuspid regurgitation is Ebstein anomaly 55 ( Figure 24-7). In this entity there is apical displacement of the septal and posterior tricuspid valve leaflets into the right ventricle and variable tethering of the anterior leaflet as well as variability in the severity of tricuspid regurgitation. A patent foramen ovale or atrial septal defect occurs in more than 50% of patients. Other associated defects include accessory conduction pathways, pulmonic stenosis, and ventricular septal defect. 56 FIGURE 24-7 Ebstein anomaly. Physical examination findings are characterized by jugular venous distention with a visible systolic v wave in 35% to 75% of patients.25–27 Hepatomegaly is present in 90% of patients, but palpable systolic pulsation of the liver is less common. Classically the holosystolic murmur of tricuspid regurgitation is heard along the left sternal border with radiation to the hepatic region and increases in intensity with inspiration because of increased systemic venous return. 26 However, the murmur is often inaudible and can be auscultated in fewer than 20% of patients with documented tricuspid valve regurgitation.25–27 In addition, many patients have atrial fibrillation, which further confounds interpretation of the characteristic respiratory variation in murmur intensity.25–27,57 As many as 80% to 90% of patients referred for echocardiography have some degree of tricuspid regurgitation. 58 Tricuspid regurgitation can be qualitatively graded with use of color-flow Doppler imaging according to the extent of the systolic color-flow disturbance in the right atrium and semiquantitatively from the density of the continuous wave Doppler echocardiography signal ( Figure 24-8A). Severe tricuspid regurgitation is characterized by a dense and dagger-shaped continuous wave Doppler signal appearance due to rapid equalization of pressures between the right atrium and right ventricle ( Figure 24-8B). Ancillary echocardiographic findings in patients with severe tricuspid regurgitation include inferior vena cava dilation of more than 2 cm and systolic flow reversals in the hepatic veins59–62 ( Figure 24-8C). FIGURE 24-8 Severe tricuspid regurgitation. The effective regurgitant orifice area can be estimated by measuring the vena contracta on color-flow Doppler imaging; a vena contracta larger than 0.7 cm indicates severe tricuspid regurgitation.24,61–63 Quantitative Doppler assessment is also feasible with use of the proximal isovelocity surface area (PISA) method, although this requires angle correction. 61 As with auscultation of the tricuspid regurgitant murmur, respiratory changes occur that may impact Doppler quantification of tricuspid regurgitation. Both the effective regurgitant orifice and the regurgitant volume significantly increase with inspiration, independent of both the severity and pathophysiology of tricuspid regurgitation and the degree of pulmonary hypertension. 64 Tricuspid valve regurgitation has an important impact on clinical outcome and survival in patients with cardiovascular disease. Mortality is higher in patients with tricuspid regurgitation regardless of ejection fraction or severity of pulmonary hypertension. 65 Severe tricuspid regurgitation following percutaneous mitral balloon valvotomy has a negative effect on survival, 66 and as in patients who have undergone mitral valve replacement, subsequent severe tricuspid regurgitation is associated with a significant reduction in exercise capacity. 67 Tricuspid regurgitation from flail leaflets is associated with an increased risk of atrial fibrillation, heart failure, need for surgery, or death. 30 The natural history of tricuspid regurgitation due to flail tricuspid valve leaflets was demonstrated in a cohort of 60 patients at Mayo Clinic, half of whom underwent operative intervention (27 tricuspid valve repair, 6 tricuspid valve replacement). In this series, operative risk was low, and symptomatic improvement was noted in 88% of operated patients. Unoperated patients experienced higher than expected mortality (4.5% yearly; P <0.01) than a matched U.S. population. Right-sided chamber enlargement, even in asymptomatic patients, was associated with a marked increase in morbidity. Unfortunately, risk of atrial arrhythmia may persist even after successful repair. The patient’s clinical status and the etiology of the tricuspid valve regurgitation determine the appropriate therapeutic strategy ( Tables 24-2 and 24-3). 24 Correctable causes should be identified and addressed. Medical management of symptomatic tricuspid regurgitation centers on treatment of right heart failure and primarily involves the use of diuretics combined with fluid and sodium restriction to manage volume status. In patients with left ventricular dysfunction, additional medical therapy may be required for management of left heart failure, but care should be taken to avoid exacerbating fatigue and hypotension related to a low cardiac output. TABLE 24-2 Management of Patients with Severe Tricuspid Regurgitation (American College of Cardiology/American Heart Association Guidelines) *24 Class I Tricuspid valve repair is beneficial for severe TR in patients with MV disease requiring MV surgery. (Level of Evidence: B) Class IIa 1. Tricuspid valve replacement or annuloplasty is reasonable for severe primary TR when symptomatic. (Level of Evidence: C) 2. Tricuspid valve replacement is reasonable for severe TR secondary to diseased/abnormal tricuspid valve leaflets not amenable to annuloplasty or repair. (Level of Evidence: C) Class IIb Tricuspid annuloplasty may be considered for less than severe TR in patients undergoing MV surgery when there is pulmonary hypertension or tricuspid annular dilation. (Level of Evidence: C) Class III 1. Tricuspid valve replacement of annuloplasty is not indicated in asymptomatic patients with TR whose pulmonary artery systolic pressure is less than 60 mm Hg in the presence of a normal MV. (Level of Evidence: C) 2. Tricuspid valve replacement or annuloplasty is not indicated in patients with mild primary TR (Level of Evidence: C) MV, Mitral valve; TR, tricuspid regurgitation. *Classification of recommendations and level of evidence are expressed in the ACC/AHA format are as follows: Class I: Conditions for which there is evidence for and/or general agreement that the procedure or treatment is beneficial, useful, and effective. Class II: Conditions for which there is conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of a procedure or treatment: Class IIa: Weight of evidence/opinion is in favor of usefulness/efficacy. Class IIb: Usefulness/efficacy is less well established by evidence/opinion. Class III: Conditions for which there is evidence and/or general agreement that the procedure/treatment is not useful/effective and in some cases may be harmful. Level of Evidence B: Data derived from a single randomized trial or nonrandomized studies. Level of Evidence C: Only consensus opinion of experts, case studies, or standard-of-care. TABLE 24-3 Indications for Intervention in Tricuspid Valve Disease (European Society of Cardiology Guidelines) *62 TR, Tricuspid regurgitation; TS, tricuspid stenosis. *Recommendation classes and levels of evidence: Class I: Evidence and/or general agreement that a given treatment or procedure is beneficial, useful, and effective. Class II: Conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of a given treatment or procedure: Class IIa: Weight of evidence/opinion is in favor of usefulness/efficacy. Class IIb: Usefulness/efficacy is less well established by evidence/opinion. Level of evidence A: Data derived from multiple randomized clinical trials or meta-analyses. Level of evidence B: Data derived from a single randomized clinical trial or large nonrandomized studies. Level of evidence C: Consensus of opinion of the experts and/or small studies, retrospective studies, registries. †Percutaneous technique can be attempted as a first approach if TS is isolated. Tricuspid valve surgery is the only treatment demonstrated to be effective for symptomatic tricuspid valve regurgitation. At our institution, tricuspid valve repair or replacement is recommended for patients with severe tricuspid valve regurgitation without important comorbidities and (1) symptomatic right heart failure (reduced cardiac output, fatigue, exertional dyspnea, diminished exercise capacity), (2) mitral valve disease or other cardiac disease that requires operative intervention, (3) progressive right ventricular enlargement or dysfunction, and (4) select asymptomatic patients, such as patients with traumatic tricuspid valve flail with severe tricuspid valve regurgitation. Tricuspid valve operation is also recommended in patients with moderate or more tricuspid valve regurgitation undergoing other cardiac surgery. The American Heart Association (AHA)/American College of Cardiology (ACC) and European Society of Cardiology (ESC) guidelines for indications for tricuspid valve repair and replacement are summarized in Tables 24-2 and 24-3; both guidelines specify the presence of symptoms or need for additional cardiac surgery as important indications.24,62 Timing of surgery for tricuspid regurgitation remains controversial, in part because of limited and heterogenous data on postoperative outcomes. 24 Reported short- and long-term mortality rates following tricuspid valve surgery are high, with up to 20% operative mortality and 50% mortality at 10 years.68–71 These rates may reflect the latent course of tricuspid regurgitation, operation for which often occurs in patients with advanced disease and heart failure. Advanced age, emergency status, associated atrial fibrillation, and pulmonary hypertension are preoperative predictors of poor outcome, 69 but heart failure and elevated right-sided filling pressures (defined by short tricuspid regurgitation duration) are among the most important determinants. 71 In a cohort of patients at Mayo Clinic who underwent tricuspid valve surgery and were stratified according to according to severity of preoperative heart failure, operative mortality was higher (18%) in patients with New York Heart Association class IV symptoms than in those with less advanced heart failure (0% for class II, 9% for class III; P = 0.02). Similarly, long-term outcomes, regardless of whether concomitant left-sided valve replacement was performed, were better in patients without advanced heart failure symptoms preoperatively. 71 These findings argue for earlier intervention, before the onset of severe right ventricular dysfunction and heart failure. Accurate imaging of the tricuspid valve anatomy prior to surgery is paramount. Although intraoperative transesophageal echocardiography may allow refinement of annuloplasty techniques to optimize outcome,72–74 assessment of the tricuspid valve with intraoperative transesophageal echocardiography is difficult owing to limited Doppler echocardiography angles of interrogation and periprocedural hemodynamic alterations that may reduce the severity of tricuspid regurgitation. A comprehensive assessment of the severity of tricuspid regurgitation is best undertaken by careful preoperative transthoracic echocardiography. 24 In the setting of tricuspid annular dilation in the absence of significant abnormalities of the tricuspid valve leaflets, tricuspid valve repair is generally the preferred approach. Singh et al, 75 comparing tricuspid valve replacement with repair in “primary” tricuspid valve disease, demonstrated that tricuspid valve repair was associated with better perioperative and mid-term event-free survival than tricuspid valve replacement, and despite increased severity of recurrent tricuspid regurgitation in patients undergoing repair, there was no difference in reoperation rates or New York Heart Association functional class during follow-up. 75 Options for tricuspid valve repair include ringed or flexible band annuloplasty, DeVega (purse-string) annuloplasty, edge-to-edge (Alfieri-type) repairs, and posterior annular bicuspidalization.76,77 Robotically assisted, minimally invasive tricuspid valve repair techniques have also been employed and may be a potential alternative. 78 Compared with a purse-string annuloplasty, ringed annuloplasty is associated with better long-term event-free survival and greater freedom from recurrent tricuspid regurgitation. 79 The degree of tricuspid valve tethering and the severity of early postoperative left ventricular dysfunction and recurrent tricuspid regurgitation are important determinants of residual and persistent tricuspid regurgitation following tricuspid valve repair.80,81 Although preoperative and postoperative pulmonary hypertension was not predictive of recurrent tricuspid regurgitation, postoperative increase in pulmonary artery pressures was a risk factor. 81 Tricuspid valve replacement is indicated for patients who have abnormal tricuspid valves not amenable to repair, including those with carcinoid heart disease, rheumatic heart disease, some patients with Ebstein anomaly, and those with recurrent tricuspid valve regurgitation after prior repair. Most commonly, tricuspid valve replacement is undertaken with a bioprosthesis, which avoids the need for long-term anticoagulation, and the durability of right-sided bioprostheses is superior to that of left-sided prostheses, likely related to lower transvalvular pressure gradients. 82 Pericardial bioprostheses are generally avoided in the tricuspid position owing to leaflet stiffness and the associated risk of obstruction. Mechanical tricuspid valve prostheses can be considered in the patient with an established indication for long-term anticoagulation, for example, concomitant mechanical left-sided prosthesis or atrial fibrillation. Although there is a risk of thrombosis or bleeding due to long-term anticoagulation with mechanical valves, several large series have reported no differences in mortality between bioprosthetic and mechanical tricuspid valves.68,70,83,84 Percutaneous native tricuspid valve replacement has been performed in animal studies. Off-label use of the Melody bioprosthetic percutaneous pulmonic valve placed in a dysfunctional tricuspid bioprosthesis has been reported in a small number of patients and has yielded promising results with reduction in tricuspid regurgitation or transvalvular gradient and clinical improvement85,86 ( Figure 24-9). Video-assisted minimal-access procedures to replace the tricuspid valve may be another alternative treatment option. 87 FIGURE 24-9 Dysfunctional tricuspid valve prosthesis treated with Melody stented valve (valve-in-valve procedure).
Diseases of the Tricuspid and Pulmonic Valves
Pathophysiology of Right-Sided Valve Disease
Response of the Right Heart to Pressure and/or Volume Overload
Principles of Diagnosis
Valve Stenosis and Regurgitation
Right Ventricular Size and Function
Tricuspid Valve Anatomy
A, Pathology image demonstrating the right ventricle (RV) with tricuspid valve (TV) in short axis. The pathologic section is oriented to replicate the transesophageal transgastric view obtained at a transducer angle of 31 degrees. Note septal (S), anterior (A), and posterior (P) leaflets. MV, Mitral valve; PV, pulmonic valve; B, Transesophageal transgastric echocardiographic image using same imaging plane (defined by the border in A) demonstrating the right ventricle with tricuspid valve septal, anterior, and posterior leaflets. (Pathologic image courtesy Dr. William D. Edwards, Department of Laboratory Medicine and Pathology, Mayo Clinic College of Medicine).
Tricuspid Regurgitation
Etiology
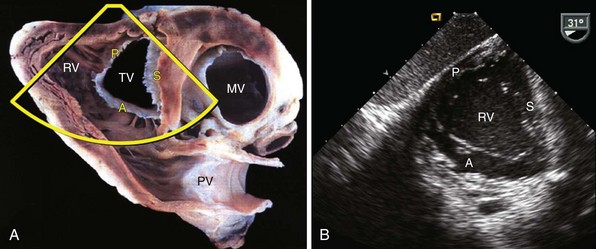
A, Color-flow Doppler image demonstrates tricuspid regurgitation related to multiple device leads. B, Zoomed view of tricuspid valve in apical four-chamber view with multiple leads (arrow) crossing the valve. C, Three-dimensional echocardiographic image of the tricuspid valve. Full volume 3D image of the heart was obtained, and image was cropped to obtain a short-axis view of the tricuspid valve, shown here from the right ventricular aspect of the valve. The device leads (arrow) are seen impinging on the motion of the septal leaflet. RA, Right atrium; RV, right ventricle; S, septal leaflet.
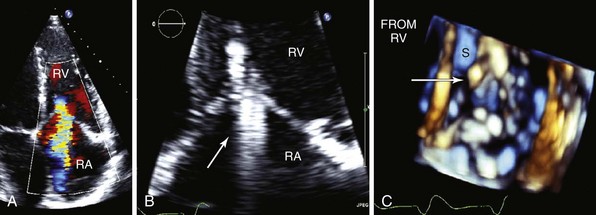
A, Parasternal right ventricular inflow view; the posterior leaflet is flail (arrow). CS, Coronary sinus; RA, right atrium; RV, right ventricle. B, Apical four-chamber view with color-flow Doppler imaging. Eccentric, laterally directed jet of tricuspid regurgitation. LA, left atrium; LV, left ventricle.
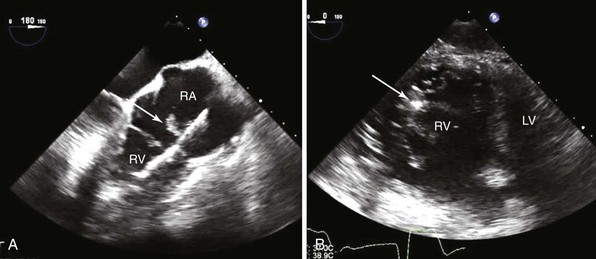
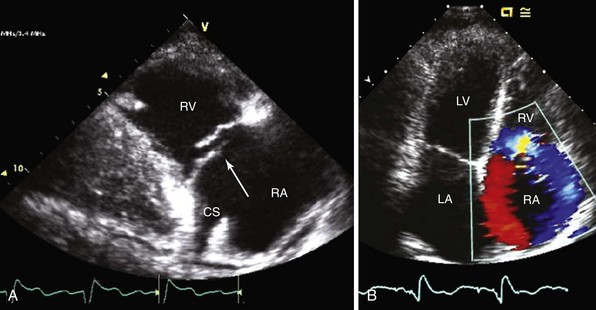
A, Transesophageal echocardiography shows vegetation (arrow) attached to the right atrial (RA) portion of the right ventricular (RV) defibrillator lead and to the posterior leaflet of the tricuspid valve. B, On transgastric short-axis image, the tricuspid valve and the defibrillator lead and vegetation (arrow) are visualized moving together with the posterior leaflet. Following device extraction, the vegetation was still attached to the posterior leaflet. LV, Left ventricle.
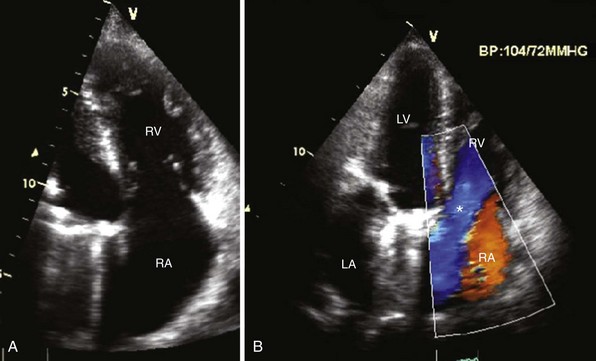
A, Apical four-chamber view demonstrates thickened septal and anterior tricuspid valve leaflets with right ventricular (RV) enlargement and dysfunction in carcinoid heart disease. The patient previously had mitral valve replacement. B, Color-flow Doppler image shows severe tricuspid regurgitation in the same patient. Note the laminar color flow (asterisk) filling an enlarged right atrium (RA). LA, Left atrium, LV, left ventricle.
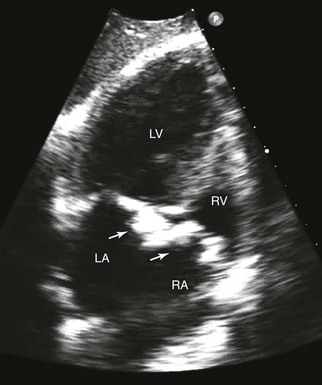
Extensive thickening and calcification of both the mitral and tricuspid annuli (arrows) and leaflets., LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
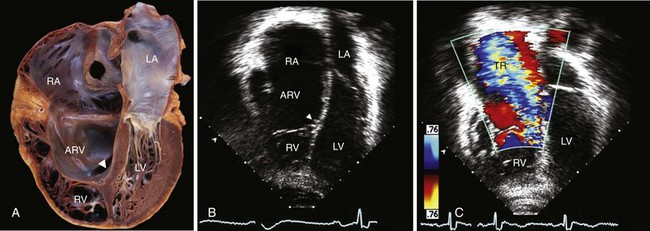
Characteristic findings are apical displacement of the septal leaflet of the tricuspid valve (arrowhead) and variable tethering of the anterior leaflet. The segment of right ventricular (RV) myocardium between the leaflet insertion and the anatomic annulus is “atrialized” (ARV), as seen in the pathology specimen demonstrated in the apical four-chamber “apex down” imaging format (A) and the two-dimensional echocardiographic image demonstrated in the same format (B). C, Because of associated valve disease, severe tricuspid regurgitation (TR) was noted on color-flow imaging, resulting in severely enlarged right heart chambers. LA, left atrium; LV, Left ventricle; RA, right atrium. (Pathology image courtesy Dr. William D. Edwards, Department of Laboratory Medicine and Pathology, Mayo Clinic College of Medicine.)
Diagnosis
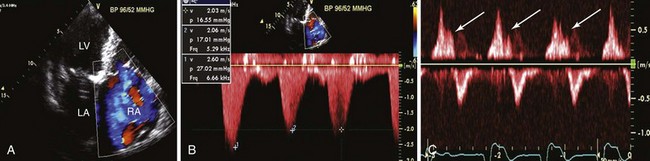
A, Apical four-chamber view. With color-flow Doppler imaging, the tricuspid regurgitant jet fills >10 cm2, or ≥30% of the right atrium (RA). B, Continuous wave Doppler signal across the tricuspid valve demonstrates a dagger-shaped signal consistent with rapid equalization of right ventricular and right atrial pressures. C, Hepatic vein pulsed wave Doppler signal with accompanying electrocardiogram tracing demonstrating systolic flow reversals (arrows), reflecting retrograde flow in the hepatic veins that can be appreciated clinically as a pulsatile liver and a v wave on the jugular venous examination. Hepatic vein systolic reversal may not be specific for severe tricuspid regurgitation when atrial fibrillation is present. LA, Left atrium; LV, left ventricle.
Natural History
Medical and Surgical Treatment
General
INDICATION
CLASS
Symptomatic severe TS
IC
Severe TS in patients undergoing left-sided valve intervention
IC
Severe primary or secondary TR in patients undergoing left-sided valve surgery †
IC
Symptomatic severe isolated primary TR without severe RV dysfunction †
IC
Moderate primary TR in a patient undergoing left-sided valve surgery
IIaC
Moderate secondary TR with dilated annulus (>40 mm or >21 mm/m2) in a patient undergoing left-sided valve surgery
IIaC
Severe TR and symptoms, after left-sided valve surgery, in the absence of left-sided myocardial, valve, or right ventricular dysfunction and without severe pulmonary vascular disease
IIaC
Severe isolated primary TR with mild or no symptoms and progressive dilation or deterioration of RV function
IIbC
Timing of Surgery
Tricuspid Valve Repair Versus Replacement
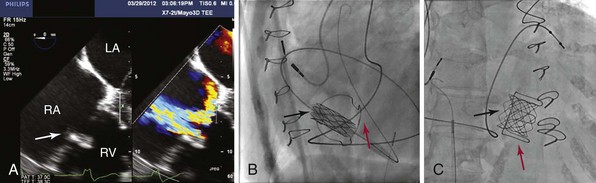
A, Transesophageal echocardiography demonstrating a transverse view of the right atrium (RA), left atrium (LA), right ventricle (RV), and tricuspid valve bioprosthesis (arrow; left panel). Severe prosthetic tricuspid regurgitation is noted on color-flow Doppler imaging (right panel). B, Fluoroscopy image, left lateral view. A catheter with the Melody valve (black arrow) is placed through the tricuspid valve bioprosthesis (red arrow). Note the dual-chamber pacemaker with a coronary sinus lead as the primary ventricular pacing lead; no right ventricular lead was placed through the tricuspid valve bioprosthesis. C, Right anterior oblique view following completed placement of the Melody valve (black arrow) with the stent fully deployed through the tricuspid valve bioprosthetic valve (red arrow).![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Diseases of the Tricuspid and Pulmonic Valves
Only gold members can continue reading. Log In or Register to continue