26

Diseases of the Pleura
Pleural Effusion, Empyema, and Pleural Biopsy
Pleural effusion refers to fluid, either serous or sanguinous, within the pleural space. Pleural effusions are usually categorized as transudates or exudates; the latter meet at least one of the following criteria: (1) pleural fluid/serum total protein ratio >0.5; (2) pleural fluid/serum lactate dehydrogenase (LDH) ratio >0.6; and (3) pleural fluid LDH two-thirds the upper limits of normal for serum.1,2 Empyema refers to pus within the pleural space; the distinction from an exudative effusion is usually obvious, but in some instances, particularly in tuberculosis, pus may be thin and watery and distinguishing it from exudative effusion may be difficult.3
The etiologies of pleural effusion are legion and a partial list is presented in Table 26–1. The exact frequency with which various causes of effusion are seen depends on the nature of the patient population and the specialty of the physician. Table 26–2 provides estimates of the relative numbers of cases of effusion of different etiology in the United States. Congestive heart failure is by far the commonest cause of pleural effusion.2 Most cases of effusion secondary to cardiac failure are not biopsied, so that in biopsy series neoplasms and infections account for the majority of cases.4–14 Markedly hemorrhagic effusions are commonly associated with trauma, surgical procedures, tuberculosis, pulmonary infarctions, benign asbestos effusion, and neoplasms; the latter cause the vast majority of spontaneous hemorrhagic effusions, and indeed account for the majority of all exudative effusions in patients over 60.1
Details of the clinical and chemical features that aid in approaching an etiologic diagnosis of effusion are presented elsewhere.1,2,15 For the pathologist examining pleural biopsies or cytologic preparations of pleural fluid, only a limited number of morphologic etiologies of effusion can be diagnosed. These include neoplasms; granulomatous reactions, which are largely tuberculosis but include fungal infections, sarcoid, and rheumatoid nodules; and to a limited extent, drug reactions.
The exact yield depends on the diagnostic technique. Tomlinson and Sahn16 summarized 14 studies reporting nearly 3026 patients during the period 1958 to 1985 and found that a diagnosis was established by thoracentesis and closed pleural needle biopsy in 57% of cases eventually proved to be carcinoma and in 75% of cases eventually proved to be tuberculosis, the latter using a combination of histologic examination and culture. Although there is variation from report to report, pleural needle biopsy and cytologic examination by themselves probably yield roughly comparable results.5,8,9,11 The combination of cytology plus needle biopsy is often better than either technique alone. Culture of the specimen also improves the yield for infectious etiologies, particularly tuberculosis, because the organisms may not always be detectable in histologic sections.4,6,7,9,17
The yield of pleural needle biopsies, both for benign and malignant underlying disease, can be improved with repeated biopsies because the pathologic processes may be widely but discretely scattered over the pleura.7,18 For example, Jimenez et al18 performed four needle biopsies in each of 84 patients and examined how the yield changed by looking at each individual biopsy and then summing the result. They found that for malignancies the yield rose from 54% with one biopsy to 89% with four biopsies.
Transudative effusions Cardiac failure Pericardial disease Nephrotic syndrome Cirrhosis Superior vena cava syndrome Meig’s syndrome Peritoneal dialysis Myxedema Exudative effusions Parapneumonic effusions and emphysema Bacterial Viral Tuberculous Fungal Pneumocystis Collagen vascular diseases, especially lupus and rheumatoid arthritis Wegener’s granulomatosis Pulmonary infarctions Neoplasms Radiation Uremia Intra-abdominal processes Acute pancreatitis Subphrenic abscess Abdominal surgery Hepatic or splenic abscess Viral hepatitis Splenic infarcts Chylous effusions Lymphangioleiomyomatosis Mediastinal malignancies (usually lymphoma) Postsurgery (trauma to thoracic duct) Yellow nail syndrome Eosinophilic effusions Idiopathic Drug sensitivity* Nitrofurantoin Dantrolene Methysergide Procarbazine Methotrexate Bleomycin Mitomycin Bromocriptine Practolol Amiodarone Minoxidil Pneumonia Pulmonary infarction Hemorrhagic effusions Neoplasms Tuberculosis Pulmonary infarcts Benign asbestos effusion Trauma Surgical procedures Pneumothorax Postcardiac injury syndrome |
*Not all drug-induced effusions show eosinophilia.
Type of Effusion | Number of Cases |
|---|---|
Congestive heart failure | 500,026 |
Bacterial pneumonia (parapneumonic) | 300,026 |
Malignancies | 200,026 |
Pulmonary emboli | 150,026 |
Viral pneumonias (parapneumonic) | 100,026 |
Cirrhosis | 50,026 |
From Broaddus and Light.2
In cases where thoracentesis and needle biopsy have failed to provide a definitive diagnosis, thoracoscopy has proven to be extremely valuable.1,10,12–14,19 Thoracoscopy is particularly useful in conditions where direct examination of the pleura allows visualization and biopsy of localized lesions, and also for specific types of tumors, especially malignant mesothelioma, where a fairly large piece of tissue is required for diagnosis (see Primary Tumors of the Pleura, later in the chapter). In addition, thoracoscopy also allows talc poudrage for pleurodesis to be performed. Menzies and Charbonneau10 reported a definitive diagnosis in 95 of 102 patients (93%) undergoing thoracoscopy and reviewed 1500 other cases from eight series in the literature; the overall diagnostic yield exceeded 90%. Later studies reported similarly high rates of specific diagnoses.12–14 As with needle biopsies, the highest yields are obtained in patients with malignancies or tuberculosis.12,14
Although there is variation from institution to institution, most studies have shown that the vast majority of neoplasms found in pleural biopsies originate in the lung and the breast1,2,5,7–10,20 (Table 26–3; Fig. 26–1). Most series also report a sizable number of lymphomas (Table 26–3). In patients with AIDS, the most common cause of pleural effusions is a parapneumonic effusion,21 but Kaposi’s sarcoma is the second leading cause and can be diagnosed in pleural biopsies.22–26
Konikov et al20 have examined the survival of patients with a positive cytologic diagnosis of pleural malignancy; their mean survival for 140 patients was 2.2 months. Patients with lung cancer had a mean survival of 2.6 months, those with breast cancer 6.1 months, ovarian cancer 7.2 months, and lymphoma 2.0 months; the latter figure would probably be considerably improved with current treatment methods.1 Occasional individuals with breast cancer survived to 3 and 4 years, whereas the longest survival for lung cancer patients was 11 months. Survival in mesothelioma is typically less than 2 years (see Primary Tumors of the Pleura, later).
After cardiac failure, infection is the next most frequent cause, overall, of pleural effusion (Table 26–2); in most instances the infection is pulmonary and the effusion is parapneumonic. Even with antibiotic treatment parapneumonic effusions are found in ~60% of pneumococcal pneumonias and 40% of all other bacterial pneumonias.1,2 Effusion is seen in ~20% of viral and mycoplasma pneumonias,27–32 and in more than 60% of cases of Legionella pneumonia.1,33,34 Pleural biopsy in bacterial, viral, and mycoplasma pneumonias is as a rule unrewarding when examined histologically; the biopsies commonly demonstrate fibrin deposition and a variable infiltrate of inflammatory cells, and proliferating mesothelial cells, but rarely bacteria or viral inclusions.
Approximately 60% of empyemas evolve from parapneumonic effusions15 (Fig. 26–2). Before the antibiotic era, most empyemas were caused by Streptococcus pneumoniae, but at present anaerobic organisms are responsible for most cases that originate with a pulmonary infection.15 Spread from other infectious foci within the chest, for example, vertebral osteomyelitis, and from intraabdominal infections, especially subphrenic abscess, may also cause both effusion and empyema.
Pleural effusions may be seen in fungal infections in nonimmunocompromised patients. In patients with acute coccidioidomycosis, pleuritic chest pain is present in up to 70%, radiographic blunting of the costophrenic angle is seen in 20%, and 2 to 6% develop substantial effusions. The latter are generally associated with a pulmonary infiltrate on the affected side extending to the pleura. Pleural biopsy demonstrates the organisms.35 Involvement of the pleura may also produce pneumothorax and empyema.36 In pleural effusion produced by Histoplasma capsulatum, histologic examination of the pleura shows typical granulomas37 containing the organisms, but pleural involvement in histoplasmosis probably occurs in fewer than 5% of cases.38–40 Pleural effusion is found in ~10% of cases of pulmonary blastomycosis15,41–43; biopsy may show necrotizing granulomas and organisms. The organisms can spread from the pleural cavity to involve adjacent tissues.36
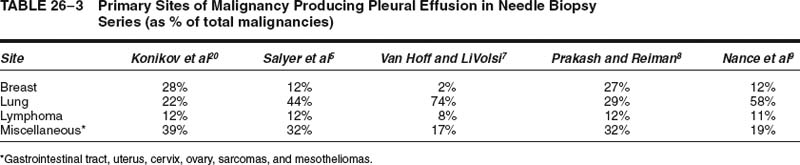
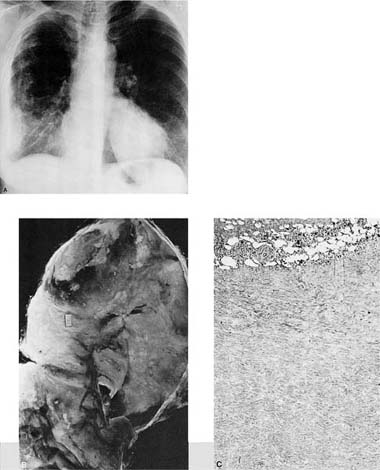
FIGURE 26–1 Pleural needle biopsy showing metastatic breast carcinoma. (A) Lower power view demonstrating skeletal muscle bundles and fibrous tissue containing inflammatory cells and tumor cells. The skeletal muscle bundles are a normal finding in this type of biopsy, but the pleura itself should normally be represented by only a single layer of mesothelial cells. (B) Higher power view showing tumor cells with inflammatory cells and desmoplastic reaction.
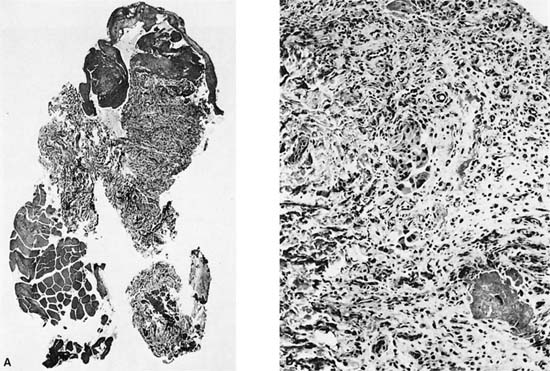
FIGURE 26–2 Gross appearance of empyema. Note the shaggy exudate on the pleural surface. The patient was an elderly male with an abscess and empyema behind an obstructing carcinoma.
Pleural involvement by fungi is more common in immunosuppressed patients. Salyer and Salyer44 found Cryptococcus neoformans in the pleura in seven of 37 patients with pulmonary cryptococcosis, most of whom were debilitated or immunosuppressed. Aspergillus infection is a rare cause of pleural effusion and is seen in several different settings:1 patients who develop a bronchopleural fistula after lobectomy for lung cancer or tuberculosis,2 patients treated in the past for tuberculosis with plombage therapy,3 and immunocompromised patients.15,45–48 In the latter the organism may appear on the pleural surface as raised yellow colonies measuring from a few millimeters to several centimeters in diameter. In infections with Aspergillus niger the pleural fluid may be brown or black.49 Fungus balls can form in the pleural space.15
Pleural infection with unusual pathogens is reasonably common in patients with AIDS. These agents have included Cryptococcus neoformans,50,51 aspergillus species,52 histoplasma species,53 nocardia species,54 and Pneumocystis carinii.55–58
In the past, tuberculosis was a very common cause of pleural effusions, and tuberculous effusions are still seen in about 3 to 4% of newly diagnosed cases in North America15,59; in many other parts of the world tuberculosis is in fact the most common cause of an exudative effusion.15 Because tuberculous effusion is associated with primary infection, it was previously considered to be a disease of adolescents and young adults;3,60 with continued increase in the number of susceptible individuals, tuberculous effusion is now also frequent in older adults. As well, mycobacterial infections are currently seen with increasing frequency in patients with AIDS.61
Clinically the disease presents with fever, chest pain, and dyspnea; sputum is not produced and cough is absent or minimal. Radiographic examination using posteroanterior (PA) films frequently fails to show a tuberculous focus within the pulmonary parenchyma, hence the older term primary pleural effusion, but the disease is nonetheless considered to result from rupture of a small subpleural granuloma into the pleural space or from dissemination of mycobacteria via pleural lymphatics during the primary infection.2,3 Hulnick et al,62 using computed tomography (CT) scans, showed that 14 of 14 patients with acute tuberculous effusions did in fact have underlying small parenchymal cavities and enlarged hilar nodes.
Thoracentesis yields a fluid that may be hemorrhagic and contains predominantly lymphocytes. Thoracentesis and needle biopsy of the pleura are excellent methods of establishing the diagnosis (Fig. 26–3); as noted above, the combination of histologic examination and culture of the biopsy and pleural fluid produced a diagnostic yield of 75% in a collected series of 3026 patients.1 Although granulomas are usually evident on histologic examination, culture may be positive even when granulomas are not seen.6,7 The pleural fluid in tuberculous effusions typically contains more than 50% lymphocytes and very high levels of protein (>50 g/L), findings strongly suggestive of the diagnosis.15 Very high levels of adenosine deaminase and gamma-interferon in the pleural fluid are also useful clues to the diagnosis.63,64
Tuberculous effusion tends to be a self-limited disease that disappears with hospitalization; however, it is important to diagnose and treat patients with tuberculous effusion, because up to 65% subsequently develop another focus of tuberculosis if left untreated.15
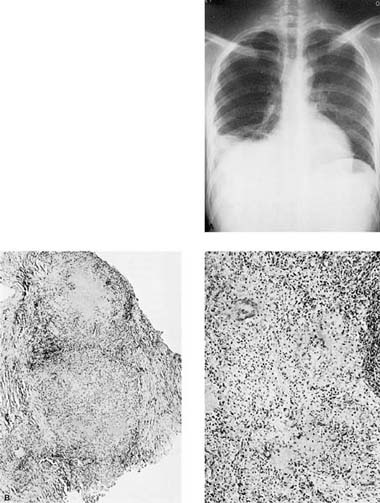
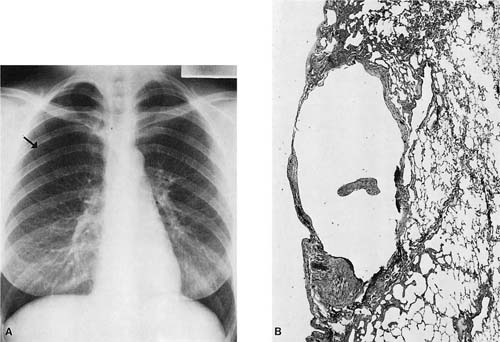
FIGURE 26–3 Tuberculous effusion. (A) Chest radiograph of a Vietnamese immigrant who presented with chest pain and a moderate right-sided effusion. (B,C) Low- and high-power views of pleural needle biopsy from this patient showing caseating granulomas. Stains for acid-fast organisms were positive.
Although sarcoid granulomas follow lymphatic routes and hence would be expected to involve the pleura in many cases, the incidence of pleural effusion in sarcoidosis is disputed. Some studies have reported finding radiographic evidence of effusion or pleural thickening in as many as 10% of patients,65 but most authors claim that clinically apparent pleural involvement is relatively rare,66 and positive histologic diagnoses even more uncommon. Sharma66 reported effusion in eight patients with sarcoidosis; needle biopsy showed granulomas in only two and nonspecific reactions in the other six. Only 2 of the 245 patients undergoing needle biopsy in the series of Van Hoff and LiVolsi7 were considered clinically to have sarcoidosis, and sarcoidosis was the final diagnosis in only two of 414 patients who had a pleural needle biopsy in the series of Prakash and Reiman.8 Obviously these figures reflect a considerable selection bias, because most cases of sarcoidosis are diagnosed by other means. The appearance of sarcoidosis in transbronchial and open/thoracoscopic lung biopsies is distinctive, and special stains are usually not necessary for such specimens, but, given the much higher frequency of tuberculosis compared with sarcoidosis in pleural biopsies, the use of special stains is advisable in this setting.
Details of the pleural findings in other, more unusual, infections such as actinomycosis, amebiasis, and parasitic infections can be found elsewhere.1,15
Pleural effusion is a common occurrence in some of the collagen vascular diseases, especially lupus and rheumatoid arthritis. Pleural effusion or pleural fibrosis (the result of organized effusion) has been reported in as many as 50% of autopsy cases of rheumatoid arthritis, but only ~5% of patients have clinically evident pleural disease during life, and then usually only in the setting of active arthritis.15,67–69 Clinically apparent pleural effusion is much more common in lupus (at least 40% of cases15,70). Needle biopsy may show rheumatoid nodules in patients with rheumatoid arthritis,7,71,72 but in my experience this is distinctly unusual, and most biopsies from patients with either lupus or rheumatoid arthritis show only a fibrinous pleuritis with organization into a thickened pleura.
A similar, totally nonspecific, histologic appearance is seen in the pleura and pericardium in the postcardiac injury syndrome. This process develops within a few weeks of cardiac or pericardial trauma, including myocardial infarction, cardiac surgery, blunt chest trauma, and pacemaker insertion. The effusion is usually serosanguineous or frankly bloody. The pathogenesis is unknown, but antimyocardial and antiviral antibodies have been observed in some patients, and an occult viral infection has been postulated as the cause.73,74
Pleural effusions may contain large numbers of eosinophils, and occasionally more than 90% of the white cells isolated from a pleural effusion are eosinophils.75 Campbell and Webb75 reviewed 101 cases of eosinophilic effusion, and found that the largest group (28%) were of unknown cause, followed by pneumonia (24%), trauma, especially pneumothorax (16%), known hypersensitivity (usually drug reactions) (14%), infarcts (6%), and malignancies (6%). Seventy-five percent of the patients had blood eosinophilia at the time of the effusion. A list of drugs known to be associated with pleural effusions is present in Table 26–1 and drug reactions producing effusions are discussed in detail elsewhere.15,76 It should be appreciated that, although many of these drugs produce an eosinophilic pleural reaction, this is not invariably true, and a drug-use history should always be elicited when dealing with effusions of unknown etiology. As well, several of these agents commonly produce pleural fibrosis, presumably as a result of recurrent effusions (see below).
The histologic counterpart of eosinophilic pleural effusion is the disease described by Askin et al77 as “reactive eosinophilic pleuritis.” The histologic picture is that of an intense histiocytic infiltration of the pleura admixed with eosinophils, giant cells, and other inflammatory cells. This appearance is nonspecific and may be seen in any of the causes of eosinophilic effusions listed in Table 26–1. The lesions described by Askin et al were found in specimens from 22 of 57 patients with spontaneous pneumothorax. Because specimens from patients with spontaneous pneumothorax commonly show some degree of fibrosis directly under the pleura, they are sometimes confused with eosinophilic granuloma (Langerhans’ cell histiocytosis). However, examination of the chest radiograph or CT demonstrates the lack of the diffuse disease, which is to be expected in eosinophilic granuloma (see Chapter 22).
Hemothorax
Hemothorax is defined as blood in the pleural cavity. The distinction between hemothorax and sanguineous effusion may be difficult; the diagnosis should be reserved for fluid, with a hematocrit at least 50% that of the blood.78 The common cause of hemothorax is trauma; spontaneous hemothorax is relatively rare, but may be seen occasionally with pleural neoplasms, with rupture of a dissecting aneurysm of the aorta, and in patients receiving anticoagulant therapy.78 Small amounts of bleeding accompanying pneumothorax (hemopneumothorax) are not uncommon. Massive hemothorax may cause shock as well as respiratory impairment.
The major complications of hemothorax are infection and organization of the blood clot to form a fibrothorax. Mesothelial tissue normally produces fibrinolysins,79 and blood is normally removed from the pleural space. Hemothorax fails to resolve in 5 to 10% of patients1 and instead organizes, eventually producing a pleural peel. In such cases decortication is usually necessary to restore pulmonary function. Empyema develops in 1 to 4% of cases of traumatic hemothorax.80 Early drainage of blood is recommended to avoid both complications.78
Chylothorax
Chylothorax is used to describe a milky, lipid-rich, chylous pleural effusion. This condition is rare and is generally associated with trauma or surgical procedures that disrupt the thoracic duct and with tumors, particularly lymphomas in the mediastinum.78 In the past spontaneous chylothorax was seen most commonly in tuberculosis. Chylothorax is also a frequent complication of lymphangioleiomyomatosis (seen in ~20% of patients81) because of lymphangiomas blocking or disrupting the thoracic duct. Chylothorax does not result in pleural fibrosis but will lead to malnutrition along with respiratory impairment. Chylothorax can be treated by repairing the duct surgically or by creating a pleuroperitoneal shunt.
Fibrosis of the Pleura
Organizing Effusions and Simple Pleural Fibrosis
The pleura shows a variety of patterns of fibrosis (Table 26–4). These may be either localized (apical caps, pleural plaques, rounded atelectasis) or diffuse. Most of the diffuse forms of visceral or parietal pleural fibrosis result from organization of recurrent effusions; the common etiologies are collagen vascular diseases, asbestos exposure, and drug reactions (Tables 26–4 and 26–5). Extensive pleural fibrosis may produce a restrictive functional impairment.
Histologically diffuse pleural fibrosis shows nonspecific changes; old lesions consist of sparsely cellular fibrous tissue, whereas active lesions demonstrate a fibrinous exudate overlying a layer of reactive mesothelial, endothelial, and chronic inflammatory cells. Occasionally such processes can be difficult to distinguish from sarcomatous or desmoplastic mesotheliomas, but a useful clue to the benign nature of the process is gradual replacement of the cellular populations by dense fibrous tissue as one moves from the active organizing surface at the pleural surface to the chest wall. This topic is discussed at more length later in the section on malignant mesothelioma.
Diffuse visceral (parietal) pleural fibrosis Collagen-vascular disease Asbestos exposure Drug reactions Any other cause of recurrent exudative effusions Fibrothorax Organization of a hemothorax Organization of an empyema (especially tuberculous) Secondary to plombage therapy for tuberculosis with escape of plombage material Severe asbestos-induced visceral pleural fibrosis adherent to pleural plaques Apical caps Pleural plaques Asbestos exposure Chest trauma Hemothorax Organized empyema Rounded atelectasis (folded lung) Pleural fibrosis secondary to asbestos exposure Pleural fibrosis secondary to renal disease Pleural fibrosis secondary to infections Pleural fibrosis secondary to trauma |
Fibrothorax
Fibrothorax refers to the formation of a thick collagenous peel that binds the parietal and visceral pleurae and effectively immobilizes a portion or the whole of the affected lung. Although the etiologies of fibrothorax are typically different from those of simple diffuse pleural fibrosis, the distinction between fibrothorax and severe fibrosis of the visceral and parietal pleurae with adhesion between the two (for example, in severe asbestos-induced pleural disease) may be moot. Patients with fibrothorax typically have a restrictive functional defect; if there is no underlying parenchymal disease, many of the physiologic changes can be at least partially reversed by decortication.78,82
In the past, fibrothorax was generally seen in cases of organizing hemothorax, organizing empyema and sometimes as a result of the treatment of tuberculosis by plombage therapy. Tuberculous empyema was a particularly common cause of fibrothorax, but identical lesions could be found complicating chronic empyema caused by other bacteria. Collapse therapy in the form of extrapleural pneumolysis and plombage was attempted with a variety of agents including paraffin, gauze, pectoral muscle, air, lucite, and methyl methacrylate, and other plastics;83,84 escape of some of these materials into the pleural space provoked a severe inflammatory reaction and eventual fibrothorax. Fibrothorax can occasionally be caused by collagen vascular disease or uremia78 but this is unusual.
Methysergide |
Ergotamine |
Ergonovine |
Bromocriptine |
Practolol |
Oxyprenolol |
Amiodarone |
Methotrexate |
Bleomycin |
Mitomycin |
From Anthony.76
Pathologically the appearance of a fibrothorax is not indicative of etiology. The lesion consists of dense fibrous tissue that often obliterates the original line of the visceral pleura and extends down into the interlobular septa and is also adherent to the parietal pleura (Fig. 26–4). Calcification or ossification may occur in long-standing cases and produce spectacular and bizarre radiographic appearances. Microscopically, the process typically extends into the chest wall fat and sometimes the chest wall muscle.
Apical Caps
Apical caps are small localized fibrous scars found over the apex of the lungs. They are most commonly encountered at autopsy but occasionally produce radiographically visible lesions that are mistaken for neoplasms and resected.85 Apical caps are usually bilateral, and on section the fibrosing process often extends shallowly into the underlying lung. In the past they have been ascribed to old tuberculous infection.86 However, such lesions are still commonly encountered at autopsy despite the markedly decreased incidence of tuberculosis and at present are typically found in lungs that have no other stigmata of tuberculosis. It has also been suggested that apical caps reflect accumulation of dust-laden macrophages and subsequent fibrosis in the apex of the lung,87 and that they are the residua of nontuberculous infections, particularly viral infections, which for unclear reasons produce apical scarring.86 More recently Yousem85 has proposed that they are secondary to ischemia. An alternate possibility is that they represent the residua of subclinical ruptured blebs of the type that produce spontaneous pneumothorax (see below); certainly the morphologic features are very similar to those found in pleurectomy specimens for spontaneous pneumothorax, with the exception that apical caps ordinarily show no active inflammation.
Pleural Plaques
Pleural plaques are discrete, raised collagenous structures with smooth or knobbed surfaces (illustrated in Chapter 24). Most pleural plaques are caused by asbestos exposure, and with this etiology are typically found over the lower lung zones on the parietal pleura and over the superior surface of the diaphragm. Other causes of pleural plaques include trauma to the chest, organization of a hemothorax, and old empyema. In contrast to asbestos-induced plaques, these lesions are usually unilateral and may be upper zonal. Rarely, identical structures are found on the visceral pleura or pericardium.
Occasionally pleural plaques take on a rounded or nodular appearance and may be mistaken radiographically for neoplasms and resected.88
Rounded Atelectasis
This lesion is also known as folded lung, shrinking atelectasis, and Blesovsky’s syndrome. The term is used to refer to entrapment of a portion of the immediately subpleural lung caused by adhesion to overlying pleural fibrosis or pleural plaques. With time the overlying fibrotic process scars and shrinks, twisting the underlying lung and forming a pseudotumor. The process typically appears radiographically as a rounded mass lesion (hence the name) abutting the pleura. Clinically, these lesions are frequently mistaken for carcinoma, but a CT scan often reveals bronchi and vessels that bend and then converge toward the lesion in a so-called comet-tail.89,90
Gross examination of a resected surgical specimen or autopsy lung typically demonstrates scarring of the pleura with retraction and rounded collapse of the underlying lung (illustrated in Chapter 24). If a thoracotomy is performed, the surgeon may dissect the pleural scar, and reexpand the lung, so that the “mass” disappears and pathologically little except pleural scarring is evident.
The vast majority of cases of rounded atelectasis are caused by exposure to asbestos,89 with subsequent pleural fibrosis and/or plaque formation (see Chapter 24). However, rare cases have been reported after chest trauma with hemothorax,89 infection,91 uremia,92 after therapeutic pneumothorax,89 and secondary to effusion of unknown etiology.89
Pneumothorax
Pneumothorax is defined as the presence of air in the pleural space. Pneumothorax can be divided into traumatic, iatrogenic, and spontaneous causes; the latter are further subdivided into secondary spontaneous pneumothorax, when there is known disease in the underlying lung, and primary spontaneous pneumothorax, when there is normal or essentially normal underlying lung. When the pleural tear acts as a check valve and air accumulates under pressure in the pleural space, the condition is termed a tension pneumothorax. A list of the causes of pneumothorax is presented in Table 26–6. Recent overviews of the topic are available elsewhere.93,94
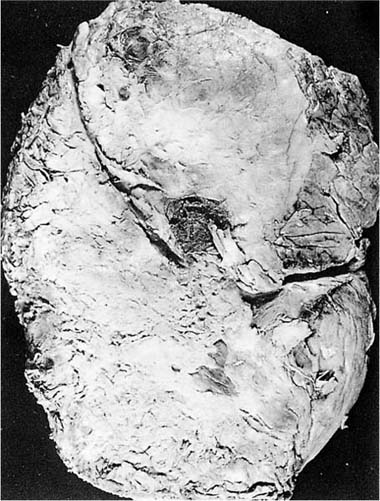
FIGURE 26–4 Fibrothorax. (A) Chest radiograph showing fibrothorax that developed as a result of a tuberculous empyema more than 20 years in the past. (B) Example of a decortication specimen showing thick fibrous pleural peel. (C) Microscopic appearance of fibrothorax. Note dense collagen with scanty inflammatory cells.
Traumatic pneumothorax |
Iatrogenic pneumothorax |
Closed pulmonary or pleural biopsy |
Mechanical ventilation |
Thoracotomy |
Placement of subclavian vein catheter |
Primary spontaneous pneumothorax |
Secondary spontaneous pneumothorax |
Adult respiratory distress syndrome |
Asthma |
Catamenial pneumothorax (thoracic endometriosis) |
Cystic fibrosis |
COPD (emphysema) |
Ehlers-Danlos syndrome |
Eosinophilic granuloma of lung |
Esophageal rupture |
Hyaline membrane disease of the newborn |
Lymphangioleiomyomatosis |
Marfan’s syndrome |
Meconium aspiration |
Necrotizing infections, especially tuberculosis and Pneumocystis carinii pneumonia (in AIDS patients) |
Malignant neoplasm, primary or secondary |
Pneumoconioses with associated interstitial pneumonia |
Pulmonary infarction |
Sarcoidosis |
Usual interstitial pneumonia |
Traumatic pneumothorax is seen in accidents or events that result in either trauma to the lung or disruption of the integrity of the chest wall or sometimes the diaphragm and intestines. Iatrogenic pneumothorax is seen invariably with thoracotomy, and with variable frequency after percutaneous biopsy procedures. Gaensler95 cites values as high as 5l% of cutting needle biopsies, 43% of trephine needle biopsies, and 5.5% of transbronchial biopsies. Placement of a catheter in a subclavian vein sometimes results in pneumothorax.92 Mechanical ventilation is another frequent iatrogenic cause of pneumothorax. The pathologic findings in these cases reflect the disease, if any, in the underlying lung; the clinical features are those of spontaneous pneumothorax with the addition of changes produced by the underlying lung disease. Hemothorax may complicate any of the situations listed above.
The majority of cases of spontaneous pneumothorax are of the primary variety.96 Estimates of the incidence of primary pneumothorax range from 7.4 to 18 cases/100,026 in men and 1.2 to 6 cases/100,026 in women,93,94,97,98 with a peak incidence between the ages of 10 and 30. Cases are rare over age 40.93,96,99–101 These figures may be underestimates because there are many apparently subclinical cases. More than 90% of patients with primary spontaneous pneumothorax are smokers or ex-smokers.94
The incidence of secondary pneumothorax has been estimated at 6.3 cases/100,026 in men and 2.0 cases/100,026 in women.97 Throughout the 19th century and the first third of the 20th century, spontaneous pneumothorax was believed to result almost invariably from tuberculosis; Biach102 in 1880 reported 918 cases, of which 715 were thought to be associated with tuberculosis. Until the 1930s, patients presenting with spontaneous pneumothorax were generally treated as if they did have tuberculosis. In 1932 Kjaergaard103 showed that in fact most cases of spontaneous pneumothorax in apparently healthy individuals were not of tuberculous etiology, and tuberculosis-related spontaneous pneumothorax currents account for a very small fraction of hospital admissions for pneumothorax. Sahn and Heffner’s93 review concluded that the most frequent causes of secondary pneumothorax were chronic obstructive pulmonary disease (COPD) and Pneumocystis carinii pneumonia related to HIV infection, followed in order by necrotizing pneumonias, interstitial lung disease, tumors, and thoracic endometriosis. A detailed list of causes of secondary pneumothorax is provided in Table 26–6.
Despite its frequency in collected series of pneumothorax patients, pneumothorax occurs in less than l% of all cases of chronic bronchitis and emphysema, but bullous emphysema may be complicated by pneumothorax in as many as 25% of cases.96 Infiltrative lung disease in which there is parenchymal fibrosis, cyst formation, or honeycombing is not uncommonly complicated by pneumothorax. Thus pneumothorax is associated with eosinophilic granuloma of the lung (pulmonary Langerhans’ cell histiocytosis) in 15104 to 44%96 of cases and is a common presenting complaint. The incidence of pneumothorax in sarcoidosis varies from 0 to 4% (average 2%) of cases in large series;105 as a rule, this phenomenon is seen late in the disease, but rare cases of early disease presenting as pneumothorax have been reported.106 Pneumothorax is seen overall in ~5% of cases of interstitial fibrosis, whether idiopathic usual interstitial pneumonia, interstitial pneumonia associated with collagen disease, or pneumoconiosis;104 among these diseases the incidence appears to be higher in those conditions that tend to progress rapidly to honeycombing. Lymphangioleiomyomatosis has a high rate of pneumothorax, and particularly of repeated pneumothorax; current data suggest that 50% of patients have a pneumothorax at first presentation and 80% develop a pneumothorax at some point.81
Infectious diseases that produce necrosis or cavitation are also relatively common causes of secondary pneumothorax. Pneumothorax occurs in 1 to 2% of hospitalized patients with AIDS,107 usually secondary to a cavitary necrotizing form of P. carinii infection,107–113 and occasionally secondary to infection with Mycobacterium tuberculosis. In this setting pneumothorax is associated with a poor prognosis.107
Fishberg114 cites pneumothorax rates of 0.73 to l3% for patients in hospital who have tuberculosis, and Wilder et al’s115 figures are 1 to 3%. More than 90% of these patients have cavitary disease. Rupture of tuberculous foci into the pleural space may produce empyema as well as pneumothorax.
Malignant neoplasms occasionally produce a pneumothorax; Dines et al116 recorded 10 instances in 1143 patients with pneumothorax at the Mayo Clinic. The pneumothorax may occur without treatment or as a result of treatment, and on rare occasions may be the first indication that a neoplasm is present. Pneumothorax has been reported with bronchogenic carcinoma, metastatic soft tissue sarcomas, lymphomas, metastatic germ cell tumors, metastatic osteosarcomas, and malignant mesothelioma.116–120
Pneumothorax is a fairly common event in newborn infants. It has been reported that some form of pulmonary air leak is seen in 27% of newborns with hyaline membrane disease, 41% of those with meconium aspiration, and 10% of those with transient tachypnea of the newborn, as well as in apparently healthy infants.121
Catamenial pneumothorax consists of spontaneous pneumothorax, almost always on the right side, occurring 48 to 72 hours after the onset of menses. Fewer than 100 cases have been reported.122,123
Clinical and Radiographic Features
Both clinical and radiographic findings depend on the presence or absence of coexistent pulmonary disease, and on the presence or absence of tension pneumothorax. As mentioned above, some patients are asymptomatic and the process may be discovered incidentally. The vast majority of patients present with chest pain and dyspnea; the latter tends to be most severe in those with the largest pneumothoraces. Hemoptysis, weakness, and syncope may also occur, particularly in patients with hemopneumothorax. Physical examination typically reveals a hyperresonant percussion note on the affected side, and breath sounds are diminished or absent. However, patients with a small pneumothorax may have a normal physical examination.1 Bilateral pneumothorax is found in only ~2% of patients.124
Roentgenographic examination usually clearly reveals the pneumothorax separating the lung from the chest wall; in those without adhesions or other underlying lung disease, the margin of the lung appears as a thin outwardly convex line (Fig. 26–5A). In emphysematous patients with large bullae, physical examination may be misleading and roentgenographic examination required to determine the diagnosis.125
Etiology and Pathogenesis
Secondary Pneumothorax
It is clear from studies of patients on artificial respiration that many different disease processes may weaken the structure of the pulmonary parenchyma and pleura.126 Conceptually, these may be divided into diseases that produce increased intrapulmonary pressure, diseases that produce thin or thick-walled cysts, and diseases that produce actual destruction of parenchyma. The first two factors probably operate together in most instances. It is possible to produce pneumothorax from high pressure alone in relatively normal lungs, for example, in asthma, but more typically pneumothorax is seen in the context of chronic airflow obstruction in patients with emphysema who have both high intrapulmonary pressures and weakened pulmonary tissues.125 Large thin-walled bullae similarly have little mechanical strength.
Infiltrative lung diseases such as usual interstitial pneumonia, sarcoid, and eosinophilic granuloma most commonly are associated with pneumothorax when honeycombing is present; it is probable that even relatively thick-walled revised airspaces may rupture because of local air trapping. Eosinophilic granuloma (Langerhans’ cell histiocytosis) is also characterized by the formation of cysts (see Chapter 22), a feature that probably accounts for the high incidence of pneumothorax in this disease.
The presence of pneumothorax with miliary tuberculosis or with early sarcoidosis, both relatively uncommon events, has been considered to represent fusion and necrosis of subpleural granulomas with rupture into the pleural space.115,116,127 Rupture of cavitary lesions or lesions undergoing necrosis into the pleural space is more easily understandable as a cause of pneumothorax in patients with cavitary tuberculosis,115 other necrotizing infections, and malignant neoplasms,116,118 particularly when the latter undergo rapid necrosis as a result of chemotherapy.119 In all of these diseases the lesion probably involves the pleura directly. Cavitation of infarcts with subsequent pneumothorax is a rare event128 that probably occurs by a similar mechanism.
A variety of theories have been advanced to explain the pathogenesis of catamenial pneumothorax.122,123,129 Few of these patients have pleural blebs.123 Most likely catamenial pneumothorax results from endometriosis of the pleura with local tissue necrosis and air leak at the time of menses. Evidence of pleural or diaphragmatic endometriosis has been observed at thoracotomy or thoracoscopy in only about one third of cases in older series;112,123,129 however, a more recent report described diaphragmatic holes or diaphragmatic endometrial implants in seven of eight patients on thoracoscopic inspection, and microscopic evidence of diaphragmatic endometriosis in all eight.130
FIGURE 26–5 Primary spontaneous pneumothorax in a young woman. (A) Chest radiograph of a young woman with multiple episodes of spontaneous pneumothorax. The partly collapsed lung is identifiable as a fine convex line in the upper right lung field (arrow). (B) Low-power micrograph of specimen from the same patient showing subpleural bleb with fibrous walls and surrounding fibrous reaction in lung tissue.
Primary Pneumothorax
The mechanism of primary pneumothorax has been the subject of considerable debate. Any explanation must consider not only the fact of pneumothorax itself, but also the findings that the majority of cases occur between the ages of 10 and 40, that there is a male predominance,93,94,100,101 and that there is a tendency for such persons to be of a tall, thin physique.101,131,132
Lichter and Gwynne132 suggested that the underlying process is a subclinical nontuberculous infection that affects the apices preferentially. The pathologic findings are certainly consistent with such a process, but there is no other evidence to suggest its correctness, and the incidence of pneumothorax has not appreciably changed despite a marked decrease in the incidence of tuberculosis.
The commonly accepted explanation is that the pneumothorax itself results from the rupture of small apical blebs. Studies using either thoracoscopy or CT scans have shown the presence of blebs in 76 to 100% of the ipsilateral lungs, and from 79 to 96% of the contralateral lungs.93 Blebs or bullae have also been found in more than 80% of nonsmokers with a history of pneumothorax.93 The origin of these blebs remains uncertain; they may represent congenital abnormalities. As noted, the incidence of primary spontaneous pneumothorax is greatly increased (up to 100-fold) in cigarette smokers.94 Emphysema in smokers is thought to arise from chronic low-grade infiltration of the lungs by smoke-evoked inflammatory cells, with persisting release of proteases that destroy the lung matrix. The same type of proteolytic attack might either lead to bleb formation or weaken the walls of preexisting blebs in smokers.
The pathologic findings in wedge resections have been described by Lichter and Gwynne132 and Glenn et al,133 and my own observations of pathologic material are similar (Fig. 26–5B). Lichter and Gwynne described the presence of small apical cysts ranging in size from 0.2 to l cm and surmounting an area of fibrosis measuring up to about 2 × 3 cm. The cysts were sometimes lined by mesothelial cells, and sometimes had a bare fibrous lining. The underlying tissue showed variable degrees of airspace enlargement, scarring, infiltration by chronic inflammatory cells, metaplastic change in airspace lining cells, and the deposition of pigment. The bronchi or bronchioles that were present frequently were obstructed by mucus. The small arteries demonstrated marked thickening of their walls. Lichter and Gwynne concluded, and I agree, that most if not all of the changes observed were secondary to inflammation; however, it is likely that the inflammatory process is a reaction to tearing of the pleura and is not the primary process.
The tall, thin body habitus associated with this condition has also attracted attention. Withers et al134 have suggested that rapid growth of relatively tall lungs toward the end of adolescence may not be accompanied by adequate vascular growth, thus accentuating the already existing relative ischemia of the apices and causing bleb formation as a degenerative phenomenon. Glenn et al133 surmise that air trapping of unknown etiology forms the blebs and that the relative ischemia of the region leads to their rupture.
A more intriguing notion related to body habitus is that the very weight of tall lungs causes damage. Vater et al135 have calculated the effects of lung shape and size on stresses within the lung; they point out the internal stress and surface pressure are most closely related to lung weight and proportional to lung height but not to width. Distortion of the lung by gravity causes higher negative intrapleural pressures at the apex and more alveolar distention with possible rupture of alveolar walls and formation of thin walled blebs.136 Careful measurements confirm not only that young men with spontaneous pneumothorax have a median height 3 inches taller than average,136 but that they have longer chests and greater height to width ratios than matched controls.100,137 One additional hypothesis that is raised by these observations is that the pneumothoraces and blebs result from a genetic defect in connective tissue. Documented genetic defects of this sort such as Marfan’s syndrome and Ehlers-Danlos syndrome are associated with spontaneous pneumothorax, and there are also families in which spontaneous pneumothorax is common. Sharpe et al138 have found an association with human leukocyte antigens (HLAs) A2B40 and also with the antitrypsin phenotype M1M2 in one such family.
Therapy and Prognosis
Several different therapeutic modalities are recommended, ranging from observation to suction to surgical bleb resection and pleurodesis. The exact choice of therapy depends on the clinical situation.93,94,96 Tension pneumothorax is a life-threatening condition that requires immediate attention. Patients with severe underlying respiratory disease may tolerate even a small pneumothorax badly125,128 and require placement of a large suction tube, followed by thoracotomy if permanent reexpansion of the lung does not occur. Healthy young patients with small pneumothoraces can be observed or treated by placement of a small valved catheter.93,94,139 Large pneumothoraces or recurrent pneumothoraces may be treated by thoracoscopy or thoracotomy with resection of apical bullae and mechanical pleurodesis or talc instillation.
Systemic Effects of Pleural Disease
The syndrome of inappropriate secretion of antidiuretic hormone (ADH) may be seen with conditions that produce mass lesion on the pleura. These include loculated empyema140 and some tumors, especially mesothelioma.141 Although some tumors, particularly bronchogenic carcinoma, produce and secrete ADH, the syndrome in the pleura appears to be a mechanical phenomenon, possibly related to stretching of vagal receptors in the visceral pleura and release of ADH from the pituitary.141,142 Inappropriate ADH secretion has also been associated with intrathoracic chemotherapy.143 The mesothelioma analyzed by Perks et al141 did not contain immunoreactive ADH, but the hormone was present in the serum and urine in increased concentration.
Large pleural tumors, particularly solitary fibrous tumors, may sometimes be associated with hypoglycemia, apparently through production of insulin-like growth factors.144–146
Primary Tumors of the Pleura
Malignant Mesothelioma and Other Diffuse Pleural Tumors
Epidemiology
In the first half of the 20th century, the nature of tumors that appeared to originate in the pleura was a matter of considerable controversy. Klemperer and Rabin147 used the term “mesothelioma” for primary pleural tumors as early as the 1930s. But the existence of true mesothelial tumors was still questioned even in the 1960s,148 and a variety of theories concerning supposed primary pleural neoplasms could be found in the literature:149 (1) that all of these neoplasms were actually metastases, (2) that they arose from endothelial cells of the pleural or chest wall capillaries or larger vessels, (3) that they arose from rests of lung deposited in the parietal pleura during embryogenesis, and (4) that they were actually derived from mesothelial cells. Several cases of what are, in retrospect, clearly mesotheliomas were described between 1930 and 1960,150 but it was really the report of Wagner et al151 in 1960, linking mesothelioma to exposure to crocidolite asbestos in the Northwest Cape Province of South Africa, that led to a detailed pathologic definition of this tumor.
Asbestos |
Erionite (in Turkey only) |
Idiopathic |
Pleural scarring |
? Therapeutic radiation |
? Simian virus 40 |
Table 26–7 lists the known causes of mesothelioma in humans. Many, but by no means all, cases are caused by asbestos exposure. The association of mesothelioma with exposure to asbestos and erionite, another durable fibrous mineral with roughly the same dimensions as asbestos but found only in a localized region in Turkey,152 and the presence of a sizable portion of cases with no definable etiology, have been established through numerous epidemiologic studies.153–158 There are 30 individual cases reported after therapeutic radiation,159,160 although a large epidemiologic study161 of individuals who received thoracic radiation for breast cancer or Hodgkin’s disease failed to find any increased incidence of mesothelioma, calling this association into question. A few cases of mesothelioma have been found in persons with severe pleural scarring, largely from previous plombage therapy or induced pneumothorax for tuberculosis, or chronic empyema,162,163 and I have seen a malignant mesothelioma arising in the pleural cavity in a young woman with extensive pleural scarring secondary to endometriosis. The possible, and controversial, association of mesothelioma and exposure to SV40 from contaminated polio vaccines is discussed later (see Pathogenesis). The general topic of non–asbestos-related malignant mesotheliomas has been reviewed by Peterson et al164 and Huncharek.159
The proportion of mesotheliomas associated with asbestos exposure varies considerably with the population examined. Rubino et al165 summarized 16 studies and found histories of exposure in 11 to 91% of patients, whereas in 70 South African cases reported by Cochrane and Webster,166 99% of the patients had been exposed. McDonald157 reported that, overall, by 1980, the proportion of male cases with exposure was 80%, whereas that of female cases only ~5%. Spirtas et al158 reexamined this issue using data from Los Angeles, New York, and 39 large Veterans Administration hospitals. They concluded that in men in the United States, the attributable risk of pleural mesothelioma was 88% and peritoneal mesothelioma, 58%. In women, for whom much smaller numbers of cases were diagnosed, the overall attributable risk was 23%. This study is unbiased in that the cases were accumulated from cancer registries rather than worker cohorts, and provides one of the more accurate estimates of the proportion of tumors associated with asbestos exposure.
The vast majority of cases of malignant mesothelioma are of pleural origin, with considerably lesser numbers of peritoneal tumors; the pericardium and tunica vaginalis are rare primary sites for mesothelioma.167,168 In North America the ratio of pleural to peritoneal tumors is ~9:1 in men and 2:1 in women.156 A few cohorts with very heavy asbestos exposure have shown a predominance of peritoneal tumors.
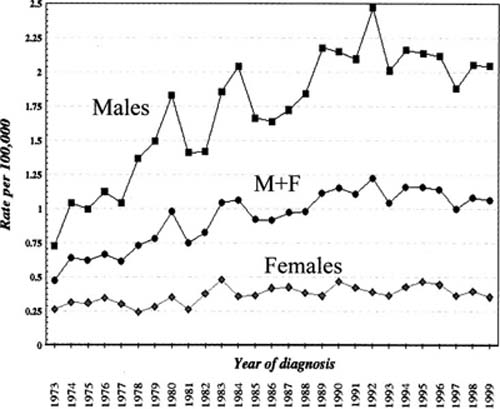
In the general population, the incidence rate of pleural mesothelioma in North America is probably in the range of two to three cases/million persons/year;169,170 this is the number seen in women (Fig. 26–6), few of whom have had asbestos exposure. In men the overall rate in North America is currently ~20 cases/million/year (Fig. 26–6)169,170 with locally higher rates in geographic regions that had large shipbuilding industries in World War II.171 Incidence rates in men have increased dramatically over the last 30 to 40 years, largely reflecting past occupational exposure to asbestos, whereas the rates in women have remained statistically unchanged169,170 (Fig. 26–6). The total number of cases in the United States was estimated at ~2500 in the year 2026, and current data suggest that that number is slowly declining169 (Fig. 26–6), a phenomenon that reflects progressive restrictions on asbestos use in the past. Much higher rates are seen in Great Britain and Australia, two countries that have extensively used crocidolite asbestos (for a discussion of the different types of asbestos, see Chapter 24): for example, for adult men in Australia in 1997, the rate was 60 cases/million/year and for adult women 11 cases/million/year.172 Case numbers in these two countries are still increasing, albeit more slowly than had been expected.173 High rates are also seen in some countries in Europe.174
Although dose–response relationships for mesothelioma have been hard to define, modeling of incidence rates from studied populations suggest that the incidence of the disease is more or less linearly related to fiber dose, and exponentially related to latency (time since first exposure).175 An additional observation that arises from these epidemiologic studies is that, unlike carcinoma of the lung, asbestos-induced mesothelioma is not associated with cigarette smoking.176
The latency period for asbestos-induced mesothelioma is usually quite long, with a range of 15 to 60 years and a mean of 30 to 40 years.177 Even in workforces with very heavy amphibole exposure, for example asbestos insulators, few tumors are seen within 20 years of first exposure and almost none with latencies shorter than 15 years;178 thus when latencies of shorter than 15 years are claimed in an individual case, evidence of past (more remote) exposures should be sought. It should also be noted that, as exposure level decreases, the latency period increases;176 thus the lower the exposure, the less likelihood that a case with a marginal latency is really caused by asbestos.
FIGURE 26–6 Incidence of malignant mesothelioma in the United States from 1973 to 1999. (From Surveillance, Epidemiology, and End Results Program.169)
Although the association of asbestos exposure with both pleural and peritoneal mesotheliomas is well established, it has become increasingly clear that there are major differences in disease potential between chrysotile and the commercial amphiboles, amosite and crocidolite (see definitions of these types of asbestos in Chapter 24). Table 26–8 shows the percentage of deaths due to mesothelioma in various occupationally exposed populations, and it is evident that chrysotile exposure is associated with a much lower mesothelioma rate than is amosite or crocidolite exposure. Indeed, in some populations with heavy exposure to pure crocidolite, as many as 18% of deaths have been caused by mesothelioma.194 A study using cohort mean fiber exposures estimated that the relative risk of pleural mesothelioma by fiber type is 1 (chrysotile):100 (amosite):500 (crocidolite).195 A recent expert panel report to the U.S. Environmental Protection Agency (EPA) also concluded that there is a two order of magnitude difference in mesothelioma risk between chrysotile and amphibole exposure.196
A corollary of these observations is that the greatest numbers of mesotheliomas are observed in persons who worked as asbestos insulators, pipe fitters, or boilermakers; in asbestos production and manufacture; and in shipbuilding or repair or in ship engine rooms, particularly during and in the 15 years after World War II.155,197,198 In all of these trades heavy amphibole exposure and chrysotile exposure was common. An increasing proportion of mesotheliomas are now seen in workers in construction trades, including plumbers, electricians, carpenters, and general laborers, where amosite use was common in the past. In contrast, mesotheliomas are rare in workforces exposed only to chrysotile (for example, textile and friction products manufacture) and there are now several reports of cohorts with no mesothelioma deaths after pure chrysotile exposure (Table 26–7). Similarly, there is no evidence of an increased risk for garage mechanics, despite exposure to chrysotile brake dust.199 Amphibole asbestos–induced pleural mesotheliomas also occur as a result of household contact exposure such as washing asbestos-contaminated work clothes.
Exposure Group | % of Deaths |
|---|---|
Chrysotile cement plant179 | 0 |
Chrysotile friction plant180 | 0 |
Chrysotile friction plant181 | 0 |
Chrysotile cement plant182 | 0.05 |
Chrysotile textile factory workers183 | 0.3 |
Canadian chrysotile miners and millers184 | 0.5 |
Balangero chrysotile mine185 | 0.5 |
Crocidolite/chrysotile friction materials factory workers186 | 0.6 |
Anthophyllite miners187 | 0.8 |
Chrysotile/amphibole textile factory workers188 | 1.3 |
Australian crocidolite miners189 | 3.3 |
Amosite factory workers190 | 4.6 |
Insulation workers (amosite/chrysotile exposure191 | 9 |
UK crocidolite-containing gas mask assemblers192 | 10 |
Canadian crocidolite-containing gas mask assemblers193 | 16 |
Workers manufacturing crocidolite cigarette filters194 | 18 |
In the past, the question of whether chrysotile asbestos induced mesotheliomas at all in humans had been debated. The data are somewhat confusing because many factories that supposedly only used chrysotile turned out, on closer investigation, also to have used small amounts of amphibole,200 and in fact mineralogic analysis of the lungs of workers from such plants indicates widespread exposure of the workforce to amphiboles,200,201 thus suggesting that the few reported mesotheliomas may have been caused by the amphibole exposures. But mineralogic analysis of the lungs of workers with mesothelioma from the chrysotile mining townships of eastern Quebec has revealed only chrysotile and its natural contaminant, the amphibole tremolite,202 thus leaving no doubt that chrysotile ore exposure, when high enough (see later) can produce mesothelioma in humans. Interestingly, these data also raise the possibility that the tremolite contaminant, rather than the chrysotile fibers themselves, may be the actual cause of the tumors,202,203 and this idea is also supported by the observation that the mesothelioma incidence in Quebec miners is highest in the mines with the most tremolite contamination.203
It is important to note that chrysotile-induced mesotheliomas only occur with very high exposure.203–205 Mineralogic analysis of lung fiber content has shown that in fact such individuals have mean fiber burdens equal to or greater than those found in chrysotile miners with asbestosis, in contrast to the situation for amosite- or crocidolite-induced mesotheliomas, where tumors appear at fiber burdens much lower than those that produce asbestosis.202,206 A recent study of women living in the Quebec mining townships and exposed historically to considerable air contamination from mining and milling operations found that mesotheliomas developed at an average cumulative exposure of ~200 fibers per cubic centimeter years (f/cc-yr),207 a value well up into the range associated with asbestosis.208 Because it is agreed by all that any recent regulatory standard for chrysotile exposure will not lead to asbestosis,209 these findings imply that chrysotile-induced mesothelioma is in fact a historic problem.
Peritoneal mesotheliomas differ from pleural mesotheliomas in that they only occur with very high exposure,195,198,210,211 and only with exposure to amosite and crocidolite asbestos.210,211 Chrysotile does not cause peritoneal mesothelioma, probably because low durability (see Pathogenesis , later, and Chapter 24) prevents chrysotile fibers from reaching the peritoneum in any significant dose.
The data just discussed are derived from and apply to populations with occupational exposure to asbestos. There has been considerable controversy in the United States about the effects of very low level asbestos exposure in public buildings in which asbestos has been used for insulation and fireproofing and the potential of such exposure to induce both carcinoma of the lung and mesothelioma.212 The exposures in question are usually only to chrysotile and are on the order of 0.0262 f/cc,212 1026- to 10,026-fold lower than the occupational standard used in different countries, and still several more orders of magnitude lower than the exposures that have led to asbestosis, carcinoma of the lung, and mesothelioma in occupationally exposed cohorts.
A variety of evidence suggests that in fact such low-level exposure poses no risk.154,155,157 The evidence is now quite strong that, as noted in Chapter 24, the association of asbestos exposure and carcinoma of the lung is really the association of asbestosis and carcinoma of the lung. Given that the development of asbestosis from chrysotile requires an exposure of at least 100 to 200 f/cc-yr,208 it is clear that building exposure will never lead to asbestosis, and hence will not produce an increased risk of lung cancer. As well, as noted above, chrysotile-induced mesothelioma only develops with high level, usually occupational, exposure.
These observations notwithstanding, it is possible to calculate theoretic risks by extrapolating incidence data from high-level occupational exposure to the levels of asbestos found in buildings. Such calculations produce lifetime mesothelioma risks on the order of 4 per million persons; this translates to an excess of 10 mesothelioma deaths in the United States per year;213 moreover, these calculated risks are far below established real risks, such as smoking, driving to work, bicycling, and even eating peanut butter (which may contain small amounts of the carcinogen aflatoxin).66 Thus there is little to support the idea that very low level exposure to asbestos poses any real risk to health.
Lastly, it is important to emphasize that, as indicated above, mesotheliomas occur both in persons who have had asbestos exposure and those who did not, and a history of exposure to asbestos or lack of exposure is important in assigning causation to a given tumor. However, a history of exposure to asbestos should play no role in diagnosis; diagnosis depends only on the pathologic findings, as it does with any other tumor.
Pathogenesis
Asbestos and Other Fibers
Some possible molecular and physical mechanisms by which asbestos induces both carcinoma of the lung and mesothelioma are discussed in Chapter 24. However, the mechanisms behind asbestos-induced carcinogenesis in the bronchial tree and the pleura/peritoneum are probably quite different; in particular, mesothelial cells are much more sensitive to the effects of asbestos, and asbestos appears to function as a compete carcinogen in the serosal membranes,214 whereas the weight of the evidence suggests that asbestos is a co-carcinogen with cigarette smoke and requires the presence of asbestosis for the development of bronchogenic carcinomas (see Chapter 24).
Molecular studies have shown several mechanisms that might be important in the pathogenesis of asbestos-induced mesothelioma. Asbestos fiber uptake by mesothelial cells is associated with the generation of oxidants, notably H2O2 and hydroxyl radical215 and oxidants can produce direct DNA damage.216 Oxidants such as H2O2 can themselves upregulate some of the cell proliferation pathways described below.217
Uncontrolled cell proliferation by definition is a feature of mesotheliomas, and asbestos appears to drive mesothelial cell proliferation in a variety of ways. In vivo mesothelial cell proliferation appears very early after intratracheal asbestos instillation, even before fibers are found close to the pleura;218,219 it has been suggested that this response reflects intrapulmonary or pleural production of hepatocyte growth factor (HGF) and keratinocyte growth factor (KGF),220 both agents that drive mesothelial cell proliferation. In tissue culture models, long amphibole asbestos fibers, but not short fibers or nonasbestos analogues, are able to directly activate the epidermal growth factor (EGF) receptor at the cell surface,221 and activation of the EGF receptor in general appears to be a reproducible property of fibers of other types, for example erionite, that cause mesothelioma.222 It appears that asbestos-induced activation of the EGF receptor leads to phosphorylation of the mitogen activated protein kinases, specifically ERK1 and ERK2, and this is followed by increased expression of the protooncogenes c-fos and c-jun, with subsequent upregulation of cell proliferation through the AP-1 signaling pathway. This process is oxidant dependent.215,216
Interestingly, cell lines derived from asbestos-induced mesotheliomas spontaneously secrete transforming growth factor-α (TGF-α), a normal activating ligand of the EGF receptor, thus leading to an autocrine pattern of cell proliferation. Platelet-derived growth factor (PDGF) A and B are also produced at high levels in mesothelioma cell lines and probably function as autocrine growth factors as well.216 Because of these observations, specific blockage of the EGF and PDGF receptors is being investigated as an approach to the therapy of mesothelioma.223
Several genetic alterations have been found in human mesotheliomas.224 There is no consistent chromosomal abnormality in every tumor, but deletions of all or part of a chromosome are common; the single most frequent finding is loss of a copy of chromosome 22. These chromosomal losses appear to be responsible for loss of several tumor-suppressor genes, particularly the CDKN2A locus, which encodes the tumor-suppressor products p16tNK4a and p14ARF, proteins that prevent cell proliferation by affecting p53- and retinoblastoma (RB)-dependent pathways. Abnormalities or loss of NF2, the neurofibromatosis type 2 tumor-suppressor gene, is also very common in mesotheliomas.
In experimental models, fiber size is crucial to carcinogenicity in the serosal membranes. Stanton et al225 injected fibers into the pleural cavity and found that long thin (i.e., high aspect ratio) fibers were much more powerful mesothelial carcinogens than were short thick fibers. In the Stanton experiments fibers longer than 8 μm with widths narrower than 1.5 μm were the most carcinogenic, but fibers with lengths greater than 4 μm and widths of less than 0.25 μm were also effective. The Stanton hypothesis has been confirmed in animal inhalation experiments using size-separated fibers; in these studies only a small number of mesotheliomas were found with either amosite or chrysotile preparations containing few fibers longer 5 μm, whereas preparations containing numerous long fibers induced much greater numbers of tumors.226,227
There is some support for the importance of fiber length from human studies. The tremolite that is a natural amphibole constituent of Quebec chrysotile ore is a relatively short thick fiber compared with commercial amosite or crocidolite,202,206 and, if one assumes that “chrysotile-induced” mesotheliomas in humans are caused by the tremolite component (see Epidemiology, earlier), then the differences in mesothelioma incidences by fiber type do correlate with fiber size. By contrast, the tremolite that contaminates some vermiculite deposits, such as that at Libby, Montana, is a much longer and thinner fiber than that in chrysotile ore, and is a potent mesothelial carcinogen,228 and the relatively long, thin tremolite that contaminates volcanic tuft used as whitewash for houses in some areas in Turkey produces high rates of mesothelioma.229
Rogers et al230
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree