22

Chronic Airflow Obstruction
The first description and illustration of enlarged airspaces was by Ruysh1 in 1721, followed by an illustration of an emphysematous lung by Baillie2 in 1799. Laennec,3 however, is credited with relating enlarged airspaces to the clinical syndrome of emphysema, and recognizing its association with chronic bronchitis. Asthma was distinguished from the general group of “dyspnea” by Floyer,4 who recognized that it could be produced by food or substances in the environment, but the best early treatise was by Salter,5 who was able to comprehensively describe asthma, in addition to suggesting that it was due to bronchospasm.
Airflow can be obstructed in the lung at one or many sites, usually categorized as large airways (bronchi), small airways, (bronchioles), and the pulmonary acinus. Thus, the patient with airflow limitation may have a wide spectrum of airway and parenchymal lesions. Terminology is important: chronic airflow obstruction is a broad term referring to the multiple states that can produce a decrease in the physiologic tests for airflow. By contrast, the term chronic obstructive pulmonary disease (COPD) is usually used when one is referring to decreased airflow due to cigarette smoking. As will become apparent, the mechanisms of airflow obstruction vary from disease to disease, although inflammation, in its most general sense, is common to all.6
A new definition of COPD has been formulated by the Global Initiative on Obstructive Lung Disease (GOLD).7 It states that COPD is “a disease state characterized by airflow limitation that is not fully reversible. The airflow limitation is usually progressive and associated with an abnormal inflammatory response of the lungs to noxious particles and gases.” GOLD categories of airflow obstruction have been formulated, with the categories based on alterations of the forced expiratory volume in 1 second (FEV1) and forced expiratory volume (FEV)/forced vital capacity (FVC) ratio. The term COPD generally includes emphysema and tobacco-associated small airways bronchiolitis. Chronic bronchitis may or may not be found in patients with airflow limitation. Asthma is simplistically characterized by reversible airflow obstruction, whereas in patients with bronchiectasis airflow limitation is secondary to the distortion of the airways accompanied by mucus and inflammatory debris in the airway lumens.
Emphysema
Definition
Emphysema is defined in strictly anatomic terms, necessitating knowledge of the normal anatomy of the lung lobule and acinus (see Chapter 1). The earliest attempt to provide an accepted and useful definition of emphysema occurred in 1959, when the Ciba Guest Symposium defined emphysema as “a condition of the lung characterized by increase beyond the normal of airspaces, distal to the terminal bronchiole, either from dilatation or from destruction of their walls.”8 Subsequent definitions9–12 differed in that destruction of respiratory tissue became a requirement: “Emphysema is a condition of the lung characterized by abnormal, permanent enlargement of the airspaces distal to the terminal bronchiole, accompanied by destruction of their walls and without obvious fibrosis.” There are compelling reasons for using the requirement of destruction in this anatomic definition in that it distinguishes emphysema from enlargement of airspaces unaccompanied by destruction, the latter being termed “overinflation.” Thus, for example, the enlargement of airspaces in the opposite lung following pneumonectomy would be termed compensatory overinflation, and the enlargement of the airspaces found with aging should be considered normal.
Simple airspace enlargement |
Congenital |
Down syndrome |
Lobar overinflation |
Acquired |
Postpneumonectomy |
Aging lung |
? Starvation lung |
Emphysema |
Centriacinar emphysema (CAE) |
Panacinar emphysema (PAE) |
Distal acinar emphysema (paraseptal emphysema) (DAE) |
Airspace enlargement with fibrosis |
Irregular emphysema |
Fibrotic lung disease |
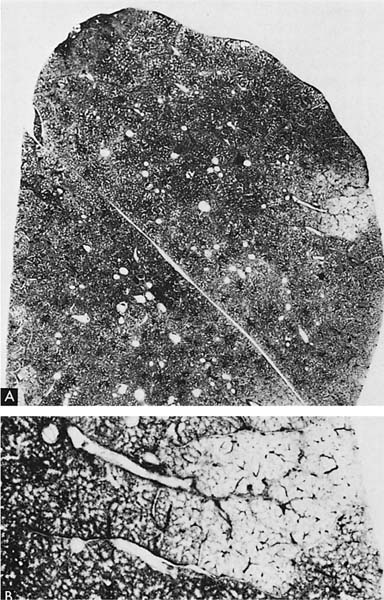
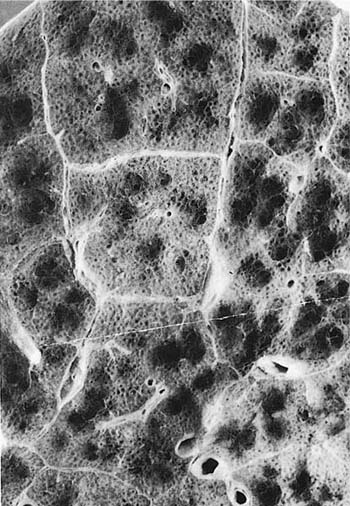
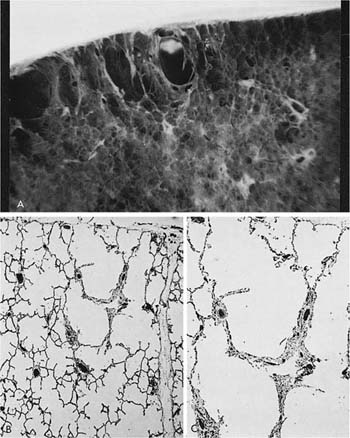
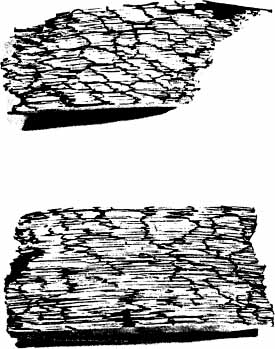
A clear distinction of emphysema from other entities characterized by enlarged airspaces was developed by the National Institutes of Health (NIH) committee report of 198512 (Table 22–1). A committee of the NIH12 proposed that destruction was present when “there was nonuniformity in the pattern of respiratory airspace enlargement so that the orderly appearance of the acinus and its components is disturbed and may be lost.” The committee recognized that emphysema was a subset of airspace enlargement, defined as “an increase in airspace size as compared with the airspace of normal lungs. The term applies to all varieties of airspace enlargement distal to the terminal bronchioles, whether occurring with or without fibrosis or destruction.” It is helpful that emphysema in its mildest form is characteristically localized, and it is the contrast in appearance between normal and abnormal lung that is used to recognize the presence of emphysema (Fig. 22–1).
Pores of Kohn/Alveolar Fenestrae
The actual existence of alveolar pores was an area of considerable debate in the late 19th and early 20th centuries13,14 but it was finally accepted that these pores (also known as vents, stomata, or fenestrae) were normal components of adult alveoli, and were responsible for collateral ventilation.14,15 An early description14 noted that the pores were situated in the intercapillary spaces in the alveolar walls, measured less than 20 μm in diameter and had sharp margins. They varied in number from two to many, with greater numbers present in those alveoli adjacent to the pleura; macrophages, fibrin, and bacteria could be found extending through the openings.
FIGURE 22–1 (A) Localized panacinar emphysema (PAE) is seen distal to an obliterated small bronchiole in the anterior segment of the upper lobe. (B) The lesion is seen better. The obliterated airways can be recognized in the area of emphysema. Emphysema is readily recognized in contrast to the normal adjacent parenchyma. (From Thurlbeck WM, Wright JL. Thurlbeck’s Chronic Airflow Obstruction, 2nd ed. Hamilton: BC Decker, 1999, with permission.)
Examination of the alveoli by either thick sections or scanning electron microscopy (SEM)16,17 suggests that holes in alveolar walls more than 20 μm in diameter are abnormal and are evidence of destruction; in non-smoker lungs less than 5% of holes are more than 10 μm in diameter and only 0.03% appear larger than 20 μm in diameter. Small fenestrae are often outlined by epithelial debris and have macrophages in the near vicinity.18,19 In subjects without emphysema, the area of the holes appears to correlate with age; fenestrations become larger and more numerous as severity of emphysema increases. In smokers the holes are larger and occupy a greater percentage of the alveolar wall in the central compared with the peripheral portion of the acinus, and this difference becomes magnified as the mean airspace size increases,20 with inverse correlations between the mean hole area or numbers of holes with pulmonary function tests.20 Comparison of lungs with centriacinar emphysema (CAE) and panacinar emphysema (PAE) by SEM demonstrates that lungs with CAE have numerous fenestrations of variable size in the emphysematous areas and variable numbers of fenestrae in the grossly normal areas, whereas lungs with PAE have only uniform and inconspicuous fenestrations.21
Destructive Index
This morphometric technique was originally developed to ascertain whether parenchymal destruction precedes airspace enlargement.22 An overall assessment of destruction, the destructive index (DI) was based on recognition of alveolar wall breaks and emphysematous destruction in the alveolar and alveolar duct spaces, and these components were respectively named DId and DIe, and expressed as the percentage of airspace examined. It was found that DI, and particularly DId, provided a good discrimination between nonsmokers and smokers and there was a larger range of DI when the mean linear concept (Lm) was in the range considered as “normal” (less than 350 μm), a finding interpreted as indicative that alveolar wall destruction precedes airspace enlargement.22,23 DI correlated with the numbers of inflammatory cells in the alveolar septa,24 suggesting that alveolar inflammation produced localized alveolar wall destruction. Other workers, however, stressed that the value of DI was in lungs with minimal emphysema, particularly in the lung parenchyma away from recognizable emphysematous holes.25,26 Although it is possible that DId represents enlarged pores of Kohn, this has not been specifically identified.
Loss of Alveolar Attachments
This technique specifically searches for destruction of the alveolar walls that tether the small airways. It is posited that loss of the attachments could explain airflow obstruction in patients with emphysema by removing radial bronchiolar support and allowing early airway closure.27,28 Several studies confirmed loss of attachments in emphysema.29–34 In general, the degree of destruction correlated with the severity of airway inflammation30,35 and with airway deformity34 and abnormalities in pulmonary function.34,36,37
Morphometric Measurements
Multiple morphometric techniques are available to assess the severity of airspace enlargement.38 These are discussed in Chapter 3.
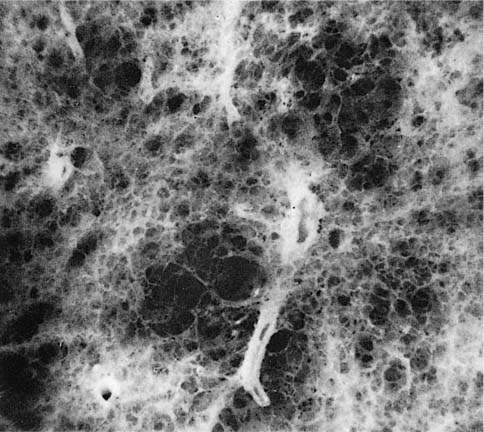
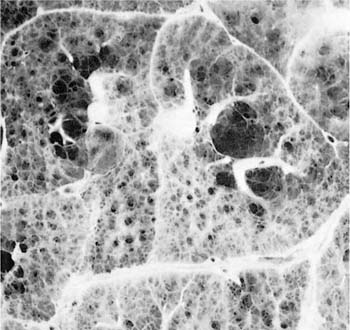
FIGURE 22–2 Several mild centrilobular emphysematous spaces are apparent. Minimal fibrosis is present and the lesions are readily recognized in contrast to the normal adjacent parenchyma. (From Thurlbeck WM, Wright JL. Thurlbeck’s Chronic Airflow Obstruction, 2nd ed. Hamilton: BC Decker, 1999, with permission)
Fibrosis
The NIH committee also considered that the presence of excess fibrosis should preclude the diagnosis of emphysema.12 Although this clearly distinguished the airspace enlargement that occurs in fibrosing alveolitis (usual interstitial pneumonia) from that of emphysema, it inadvertently also introduced confusion, in that “excess” fibrosis became interpreted as “no” fibrosis. Increased collagen has been found to be increased in most forms of emphysema.39–42 In practice, pathologists can easily differentiate between the fibrotic revised airspaces of interstitial fibrosis and the destructive “holes” of emphysema (Fig. 22–2).
Gross, Sub-Gross, and Microscopic Recognition
Emphysema can be appropriately recognized on paper-mounted whole lung (Gough-Wentworth) sections43 or by gross or dissecting microscopic examination of barium-impregnated fixative-inflated slices of lung44 (see Chapter 5 for details of preparation). A grading scheme based on whole lung slices has been long used as a reference.44 Unfortunately, despite their usefulness as a research tool, paper mounted sections are expensive, and very few institutions continue to support their use. Although a microscopic grading scheme that recognizes CAE and PAE and appears to correlate with conventional assessments of emphysema and pulmonary function tests has been developed,45 it has not been widely utilized. Pathologists should be assured that for simple diagnostic purposes, a division into none, mild, moderate, and severe should suffice.
Classification
Not only is emphysema defined in terms of lung structure, it is also classified in similar terms, and several definitions recapitulated from Chapter 1 are therefore important. The part of the lung involved is the acinus, which is defined as the unit of lung structure distal to the terminal bronchiole (final generation membranous bronchiole). An acinus is approximately spherical and measures close to 7 mm in diameter.46 The terminal bronchiole is succeeded by three orders of respiratory bronchioles that are characterized by the presence of both respiratory (alveolar) epithelium and nonrespiratory epithelium in their walls.
A lobule (secondary lobule of Miller), by contrast, is a gross term, defined as the amount of lung parenchyma that is contained within four surrounding venous septa. Lobules measure 2 to 4 cm on a side, and contain between three to five acini. The terminal bronchiole tends to be situated in the center of the lobule.
The ways in which alveolar walls are involved determine the classification of emphysema; there are four recognized patterns:
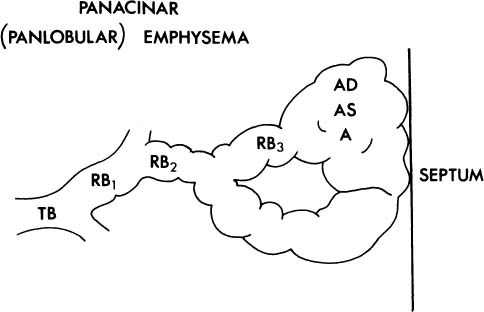
1. The acinus may be more or less uniformly involved; this is panacinar (panlobular) emphysema (Fig. 22–3).
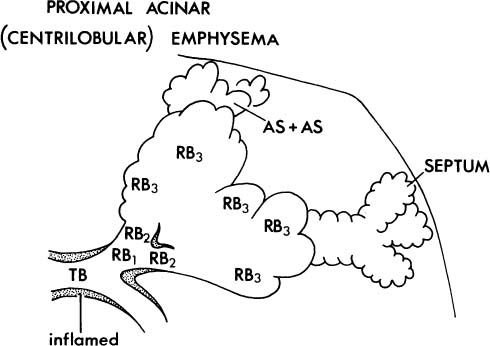
2. The proximal portion of the acinus may be dominantly or selectively or selectively involved (Fig. 22–4), and the best term for this lesion is proximal acinar emphysema, although the usual term is centrilobular or centriacinar emphysema.
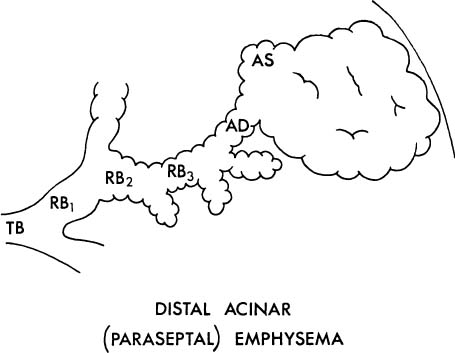
3. The proximal portion of the acinus may be normal, and the distal part (alveolar sacs and ducts) may be dominantly involved (Fig. 22–5). This is distal acinar emphysema, more usually referred to as paraseptal emphysema because the lesion is accentuated along lobular septa where the peripheral parts of the acini lie.
FIGURE 22–3 In panacinar (panlobular) emphysema, the enlargement and destruction of airspaces involve the acinus more or less uniformly. TB, terminal membranous bronchiole; RB, respiratory bronchiole; AD, alveolar duct; AS, alveolar sac; A, alveolus. (Adapted from Thurlbeck WM. Chronic obstructive lung disease. In: Sommers SC (ed): Pathology Annual. New York: Appleton Century Crofts, 1968:367–398, with permission.)
FIGURE 22–4 In centriacinar (proximal acinar, centrilobular) emphysema, respiratory bronchioles are selectively and dominantly involved. TB, terminal membranous bronchiole; RB, respiratory bronchiole; AD, alveolar duct; AS, alveolar sac; A, alveolus. (Adapted from Thurlbeck WM. Chronic obstructive lung disease. In: Sommers SC (ed): Pathology Annual. New York: Appleton Century Crofts, 1968:367–398, with permission.)
FIGURE 22–5 In distal acinar (paraseptal) emphysema, the peripheral part of the acinus is dominantly involved. TB, terminal membranous bronchiole; RB, respiratory bronchiole; AD, alveolar duct; AS, alveolar sac; A, alveolus. (From Thurlbeck WM. Chronic obstructive lung disease. In: Sommers SC (ed): Pathology Annual. New York: Appleton Century Crofts, 1968:367–398, with permission.)
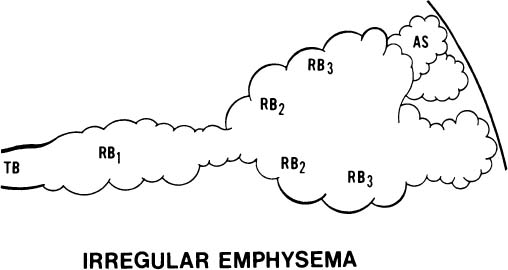
FIGURE 22–6 In irregular emphysema, the components of the acinus are randomly destroyed and enlarged. Fibrosis is a usual accompaniment of this lesion. TB, terminal membranous bronchiole; RB, respiratory bronchiole; AD, alveolar duct; AS, alveolar sac; A, alveolus. (From Thurlbeck WM. Chronic obstructive lung disease. In: Sommers SC (ed): Pathology Annual. New York: Appleton Century Crofts, 1968: 367–398, with permission.)
4. The acinus may be irregularly involved (Fig. 22–6). This is irregular emphysema or paracicatricial emphysema, so called because it is usually associated with obvious scarring.
Panacinar Emphysema
Various terms have been used to describe emphysema involving the acinus: “panacinar emphysema,”8“generalized emphysema,”47 “diffuse emphysema,”48,49 “diffuse generalized emphysema,”49,50 “alveolar emphysema,”51 and “vesicular emphysema.”52 PAE is the most accurate because it describes the lesion in terms of the acinus as is done in defining other forms of emphysema.
There appears to be some measure of agreement that PAE tends to occur rather more commonly in the lower zones and the anterior margins of the lungs,53–57 and is usually more severe at the bases58 (Fig. 22–7). It has been suggested that the earliest stage of emphysema involves the alveolar ducts, with subsequent involvement of other parts of the acinus.52,58 The precise distinction between normal and the mildest grade of emphysema is very difficult (Fig. 22–8).
In the normal lung, the multifaceted alveoli form a contrast to the larger, cylindrical conducting structures that are alveolar ducts and respiratory bronchioles. In PAE this distinction becomes lost as alveoli lose their sharp angles, enlarge, and then lose their contrast in size and shape with the alveolar ducts. As the lesions progress, there is progressive effacement of tissue and loss of the orderly arrangement of the lung until all that remains are strands of tissue. Paper-mounted sections of mild PAE demonstrate attenuation of the background of alveolar tissue, the contrast between alveoli and alveolar ducts is lost, and there is simplification of the lung structure, with formation of small box-like structures. As the process becomes worse, the alteration of lung structure becomes more obvious, with progressive loss of tissue, until little other than the supporting framework of vessels, septa, and bronchi remains (Fig. 22–9).
Examination of lung slices is best performed to contrast the lung appearance when it is immersed in a water or fixative bath, and when it is removed from the bath. When the slices are not immersed, PAE can be suspected because the lung parenchyma “falls away” from the supporting structures and protrudes slightly above them. It is important to try and contrast different areas in the slice, because the mildest grade of emphysema is usually not uniformly dispersed through the lung.
FIGURE 22–7 The frequency of centrilobular emphysema in 10 zones of the lung, roughly corresponding to segments, in 138 random necropsies (A) and in 30 cases selected because they had severe emphysema (B). In the random cases, centrilobular emphysema (CLE) is most common in the upper lobe and in the superior segment of the lower lobe. (C,D) Both severity and frequency are indicated; the higher numbers in the upper lobe and the superior segment of the lower lobe in the random cases (C) and the selected cases (D) show that the emphysema is more severe in these zones. (From Thurl-beck WM. The incidence of pulmonary emphysema with observations on the relative incidence and spatial distribution of various types of emphysema. Am Rev Respir Dis 1963;87:206–215, with permission.)
FIGURE 22–8 Examples taken from a normal lung (A,B) are compared with an example of very mild panacinar emphysema (PAE) (C,D). Note the effacement of normal lung structure; the sharp distinction among alveoli (the smallest polygonal structures) and alveolar ducts and respiratory bronchioles (the large spaces) is lost in mild PAE. (From Thurlbeck WM. Pulmonary emphysema. Am J Med Sci 1963;246:332–353, with permission.)
Histologic examination (light microscopy using paraffin sections) is a sensitive method of recognizing PAE; PAE that appears mild with a dissecting microscope or in paper sections, or even when one may have doubts as to its existence, is usually obvious histologically. The histologic pattern is again one of simplification with diminishing contrast between alveoli and alveolar ducts. Indeed, it is often hard to produce adequate paraffin sections of moderate PAE, as the tissue becomes distorted in processing.
Associations of Panacinar Emphysema
α1-Antitrypsin (α1-AT) Deficiency
α1-AT deficiency is the least controversial association of PAE. Laurell and Eriksson59 noted a familial deficiency of serum α1-globulin in families in Sweden while screening large numbers of sera by electrophoresis, and they also observed that there was a high incidence of airflow obstruction in these families. Eriksson60 demonstrated that the patients who had lowered levels of α1-globulin also had markedly diminished antitryptic activity in their serum, and hence the origin of the term α1-antitrypsin deficiency.
α1-AT is the main protease inhibitor in the lung, accounting for ~90% of inhibitory capacity. It is secreted by the liver into the vasculature, and diffuses passively across the alveolar capillary membrane and into the lung epithelial lining fluid at levels ~10% of plasma levels. Normal individuals will produce ~2 g α1-AT each day; the protein has a serum half-life of 4 to 5 days with serum levels in the range of 20 to 53 μM (reviewed elsewhere61). It is secreted in response to inflammation, and is therefore classified as one of the acute-phase reactants in the biologic defense mechanism. Neutrophil elastase binds to α1-AT with great avidity,62–64 and once bound, dissociation does not occur to any extent. The active inhibitory site is centered at a methionine residue, and this has great significance, because this site is vulnerable to oxidation by free radicals,62,65,66 and oxidation markedly decreases the inhibitory capacity.64
The α1-AT gene has many mutations61,67,68 existing as greater than 70 different variants, designated in their entirety as the Pi system, and named according to their migration position on isoelectric focusing. Inheritance is via an autosomal pattern, and the alleles are co-dominant. The common allele variants are M, S, and Z, and their frequency varies significantly depending on the population studied;67,69 the majority of the other variants have no clinical significance, and only those phenotypes composed of two “at risk” alleles61,62,67,69 would place the individual at risk for the development of emphysema.
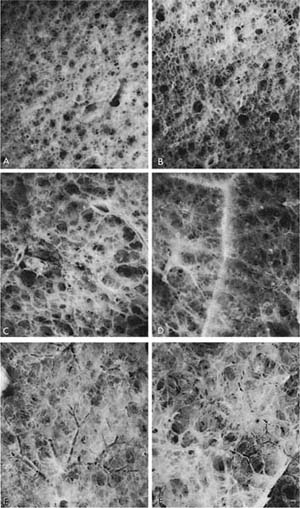
FIGURE 22–9 Different lungs have been photographed through a dissecting microscope after impregnation with barium sulfate. All are at the same magnification. (A) A 55-year-old woman. (B) A 75-year-old woman. (C–F) Examples of PAE. In the normal lung, the distinction between alveoli and ducts is clear. (See also Fig. 22–8.) In PAE, this distinction is lost and there is effacement of tissue. These are mild changes that would not be recognized on examination of the lung by the naked eye or without immersion of the samples in water and impregnation with barium sulfate. (D–F) Destruction is clearly apparent. (From Thurlbeck WM. Pulmonary emphysema. Am J Med Sci 1963; 246:332–353, with permission.)
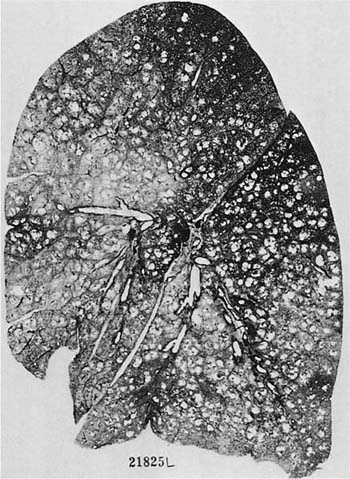
There is no doubt that the Pi type ZZ carries with it a greatly enhanced susceptibility to pulmonary emphysema; Fig. 22–10 is an example. It is estimated that 70 to 80% of the subjects with Pi type ZZ will develop pulmonary emphysema; the exceptions are usually those who do not smoke. A significant proportion of patients with clinically evident severe emphysema are Pi type ZZ, but the reported frequency has varied form 1 to 18%.70–76 Pi type ZZ is also much more common in younger patients with symptomatic emphysema, and up to 60% of such patients under the age of 40 may have Pi type ZZ. Patients with severe α1-AT deficiency have a characteristic clinical syndrome with early onset of the disease, characteristically starting with dyspnea in the fourth decade;71,77 25 to 30% of patients never develop chronic bronchitis.60,71 Pi type MZ heterozygotes may or may not develop airflow.60,72,78–80
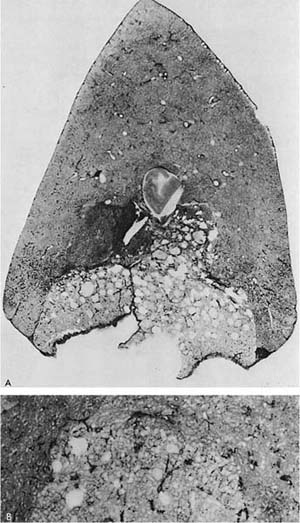
FIGURE 22–10 A patient with α1-antitrypsin deficiency has widespread PAE, which is worse in the lower zones of the lung. (From Thurlbeck WM, Wright JL. Thurlbeck’s Chronic Airflow Obstruction, 2nd ed. Hamilton: BC Decker, 1999, with permission.)
Panacinar Emphysema Associated with Bronchial and Bronchiolar Obliteration
Panacinar emphysema may be associated with, and occur as a consequence of, permanent obliteration of airways. This is usually a localized phenomenon, but widespread obliteration can also occur, generally due to infective bronchitis and bronchiolitis in childhood.81–84 When airway obliteration without atelectasis appears to affect most of one lung but to spare the other, the term Swyer-James85 or MacLeod’s86 syndrome is applied. The syndrome is also known as unilateral pulmonary transradiancy or unilateral emphysema. The radiologic findings are diagnostic. On standard inspiration films, one lung is transradiant, and the other has increased vascularity. The significance of this can be misinterpreted; often the nontransradiant side is regarded as the diseased lung. The transradiancy is due to decreased perfusion of the affected lung, not to excess air within it. When the patient breathes out to residual volume and is re-radiographed, the transradiant lung traps air. Thus the mediastinum moves to the normal side and there is gross disparity between the dimensions of the two lungs. The patients are often asymptomatic, and the abnormality may be discovered incidentally on a routine chest roentgenogram.
Atresia of the Bronchus
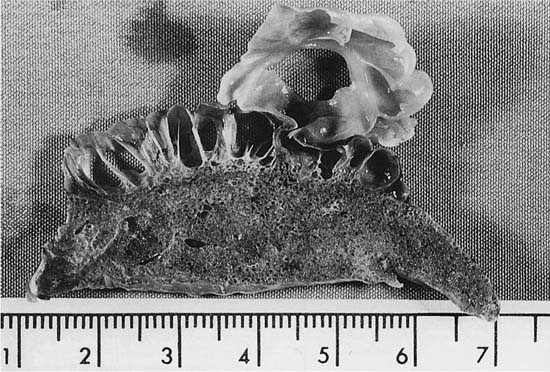
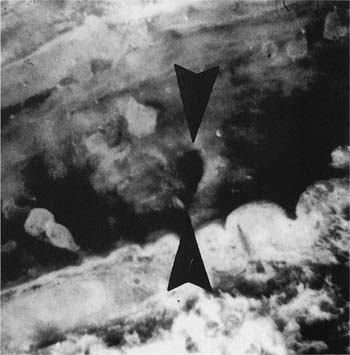
This condition is similar in some respects to the Swyer-James syndrome, but differs in that stenosis of the bronchi is congenital in nature and generally limited to one airway. The airway to the left apico-posterior segment of the upper lobe bronchus is most commonly affected,87–89 but other lobes90,91 (Fig. 22–11) and more than one lobe92 have been involved. Characteristically, there is mucus impaction and bronchial dilatation distal to the atretic bronchus. The lung parenchyma distal to the obstruction overexpands and may develop PAE.
FIGURE 22–11 Congenital atresia of the lateral segmental bronchus in the right lower lobe has resulted in a dilated proximal bronchus with mucus impaction (center of A). The contrast between normal and mildly emphysematous lung is seen best in a close-up (B), which is from a paper-mounted section of another slice of the lung.
Localized, Incidental Panacinar Emphysema
Localized, pure panlobular emphysema may found in random autopsy lungs, most frequently (up to 20%) from patients over the age of 70.93 It is generally found along the diaphragmatic surface of the lung and the lower half of the anterior surface of the lung (Fig. 22–1). It is usually slight or moderate in severity, and is invariably asymptomatic. The condition appears to be a variant of the aging lung.
Intravenous Drug Abusers
Intravenous (IV) drug abusers develop talc (the carrier for many drugs) granulomas in the lung, with dense conglomerates resembling progressive massive fibrosis of pneumonoconiosis.94 Although the granulomatous masses are most common in the upper lobes, the lower lobes may develop severe extensive PAE95,96 purportedly on the basis of elastases released by macrophages.95
Panacinar Emphysema Associated with Proximal Acinar Emphysema
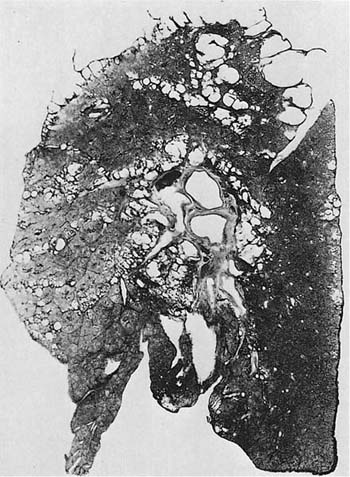
Parenchyma distal to centrilobular emphysematous spaces frequently displays a mild grade of PAE that is hard to recognize and is overshadowed by the more obvious lesion of proximal acinar emphysema. In addition, however, a very characteristic finding in patients with airflow obstruction (often gold miners who are smokers97,98) is the presence of centrilobular emphysema in the upper zones of the lung and PAE in the lower zones (Fig. 22–12). The combination of the lesions often leads to semantic disagreements about classification, but it is probably best to name both lesions and indicate which one appears to be dominant.
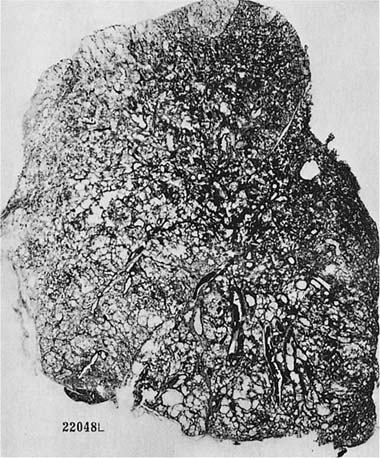
FIGURE 22–12 Severe centrilobular emphysema is seen in the upper zone, and severe PAE is seen in the lower zone of the lung shown in this paper-mounted whole lung section. (From Thurlbeck WM. Chronic obstructive lung disease. In: Sommers SC (ed): Pathology Annual. New York: Appleton Century Crofts, 1968:367–398, with pernission.)
FIGURE 22–13 Centrilobular emphysematous spaces are shown in a lung with well-demarcated lobular septa. Note that the spaces lie midway between the center of the lobules and the periphery of the lobules. Normal-appearing respiratory tissue lies between the spaces and between them and the periphery of the lobule. Note that from time to time there is encroachment and destruction of alveolar ducts and sacs (×2.5 approximately). (From Thurlbeck WM. Pulmonary emphysema. Am J Med Sci 1963;246:332–353, with permission.)
Proximal Acinar Emphysema
This type of emphysema is characterized by dominant or selective enlargement of the proximal part of the acinus, accompanied by destructive change, and thus, respiratory bronchioles are primarily involved. Although proximal acinar emphysema was once divided into centrilobular emphysema (CLE) of cigarette smokers, and mineral dust (characteristically coal) associated emphysema, there is not unanimity that the two conditions are indeed different or that they can be distinguished from each other. Indeed, the mechanisms involved in the destructive processes appear to be similar.38,99
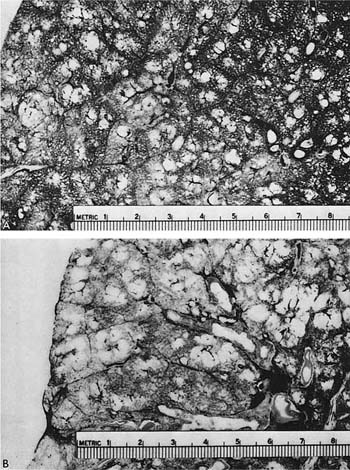
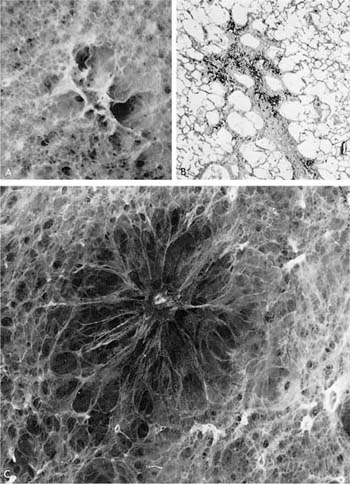
The term centrilobular emphysema was used originally because the emphysematous lesions involved the approximate center of the (secondary) lobules, or the smallest discrete portion of the lung surrounded by connective tissue septa as examined grossly, or subgrossly using a dissecting microscope. This destructive lesion of the respiratory bronchioles has several characteristic features on gross examination of the lung. One is the striking irregularity of involvement of lobules, or, indeed, the same lobule.47,100 The lesions are usually more common and become more severe in the upper zones of the lung, particularly the posterior and apical segments, and the superior segment of the lower lobe (Figs. 22–13 to 22–16).53–55,58,101,102 The walls of the emphysematous spaces and adjacent tissue characteristically contain variable amounts of black pigment.
FIGURE 22–14 In this specimen, the pulmonary arterial system has been filled with contrast medium. Note the obvious destructive nature of centrilobular emphysema. The acinar periphery appears normal (to the right), whereas at the top left there appears to be enlargement and destruction of the acinar periphery (×5 approximately).
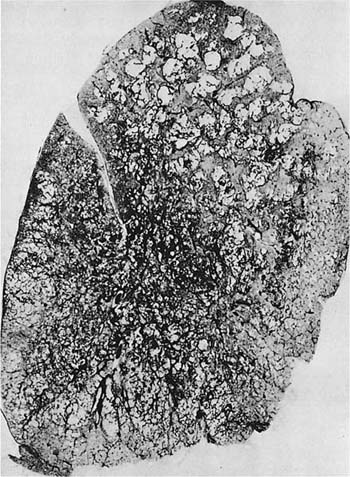
FIGURE 22–15 A paper-mounted whole lung section shows widespread centrilobular emphysema more or less uniformly distributed through the lung. (From Thurlbeck WM. Internal surface area and other measurements in emphysema. Thorax 1967;22:483–496, with permission.)
In the classical lesion, the enlarged, destroyed respiratory bronchioles coalesce in series and in parallel to produce sharply demarcated emphysematous lesions, separated from the acinar periphery (the lobular septa, pulmonary vessels, and bronchi) by intact alveolar ducts and sacs of normal size (22–13 μm). Second- and third-order respiratory bronchioles are more severely affected than the first-order respiratory bronchiole. Although the lung tissue distal to the emphysematous space is often apparently normal, there is a greater loss of alveolar surface area than can be accounted for by the centrilobular emphysematous spaces.103
Unfortunately, however, classical examples of proximal acinar emphysema are uncommon; atypical examples predominate, and interpretations of these are difficult and differ among expert observers.104,105 This is particularly true when the emphysema is severe.28,105–107 Although the peripheral part of the acinus is maintained intact in classic examples, there is often variable encroachment on the alveolar ducts, and as the spaces become larger, they tend to become confluent. However, a rim of acinar tissue can usually be seen between the spaces, and it can be determined that the dominant lesion involves the proximal part of the acinus. A lesion that may be confused with proximal acinar emphysema is isolated distal bronchiolectasis, such as is seen in early panbronchiolitis.108,109 However, these lesions tend to be uniform and mild, and the resemblance to proximal acinar emphysema is usually superficial. Histologic examination shows that the dilated structures are nonrespiratory bronchioles. The condition is uncommon and does not have the associations with chronic bronchitis and smoking so characteristic of CLE.
FIGURE 22–16 (A) A close-up of Fig. 22–15 shows the lesions clearly. (B) Larger centrilobular emphysematous spaces are shown. In some instances, the acinar periphery appears normal, whereas in others the lesions extend almost to the lobular septum. (From Thurlbeck WM, Dunnill MS, Hartung W, Heard B, Heppleston A, Ryder R. A comparison of three methods of measuring emphysema. Hum Pathol 1970;1:215–226, with permission.)
Placental Transmogrification
This unusually named entity is relatively uncommon.110–112 This lesion is thought to devolve from localized bullous emphysema in relatively young to middle-aged individuals, and it not progressive in nature. The bullous cysts contain gelatinous material with vesicles resembling placental or hydatidiform mole tissue. Histologically, there are alveolar wall remnants, and interlobular septa with multiple papillary structures both within the cysts and within the adjacent emphysematous lung parenchyma. Why bullous emphysema would devolve in such a bizarre fashion is inapparent, although the possibility of lymphatic obstruction has been suggested.
Pathogenesis of Centriacinar Emphysema
Protease-Antiprotease Balance
The most commonly accepted theory of the pathogenesis of smoking-induced emphysema is known as the proteolysis-antiproteolysis hypothesis.65,113 As noted above, mineral dust–associated emphysema may have pathologic processes very similar, if not identical, to cigarette smoke–induced emphysema.
On the antiproteolysis side of the balance, the main serine antiprotease in the lung is α1-AT. Cigarette smoke and mineral dust are known to oxidize irreversibly the Met 358 residue of α1-AT,114,115 decreasing the inhibitory capacity of the α1-AT protein ~2022 fold.64 Other antielastases include α2-macroglobulin, and low molecular weight mucus inhibitor (secretory leukoproteinase, antileukoproteinase). Antileukoproteinase is produced by mucosal cells in the upper and lower respiratory tract (reviewed elsewhere116), and is also susceptible to oxidant damage because of a methionine residue in its active site. The tissue inhibitors of metalloproteinases (TIMPs) form noncovalent complexes with the active forms of the metalloproteinases to block their activity and retard precursor activation. TIMP activity appears to be partially blocked in patients with chronic airway obstruction (CAO).117
On the proteolysis side of the balance there is evidence implicating both the neutrophil and the macrophage. The exact mechanism of recruitment of these cells is a matter of active investigation, but one consequence of the inflammatory cell influx is release of a variety of proteolytic enzymes that have the ability to digest the connective tissue matrix of the lung.118
Several studies have noted the presence of increased polymorphonuclear (PMN) leukocytes in the alveolar walls of cigarette smokers.24,119 However, this inflammatory response should also include B lymphocytes, T lymphocytes, and macrophages, and continues to be present even in severe emphysema.120 Intriguing data implicate the adenovirus in this reaction.120,121
FIGURE 22–17 (A) Distal acinar emphysema involves the acinus adjacent to the pleura (×7 approximately). (B,C) Histologic sections (B × 12 approximately, C × 25 approximately) illustrate the involvement of the distal part of the acinus. Fine fibrosis is readily apparent. (From Thurlbeck WM. Pulmonary emphysema. Am J Med Sci 1963;246:332–353, with permission.)
Examination of elastase activity in bronchoalveolar lavage (BAL) fluid has shown both serine and metalloelastase, with a predominance of metalloelastase;122–124 both levels are increased in subjects who smoke.124 Alveolar macrophages constitute the majority of inflammatory cells in the airspace125–127 and, in emphysema, these cells express elevated messenger RNA (mRNA) transcripts for gelatinase and interstitial collagenase, and secrete increased levels of collagenase into culture medium.128 New animal studies have helped to elucidate the various roles of PMN and macrophages in emphysema.129–133
There is direct evidence for elastin breakdown in smokers, with morphometric,134 electron microscopic,135 and direct biochemical analysis for desmosine and isodesmosine and hydroxyproline, or the identification of elastin-derived peptides.136–141
Apoptosis
A very early study15 suggested that the pores of Kohn appeared to occur as a result of atrophy of the ground substance near the center of the capillary mesh. Animal models have demonstrated that inhibition of vascular endothelial growth factor (VEGF) receptors is associated with an increase in apoptosis associated with the development of emphysema,142 and examination of the lungs of smokers with emphysema showed reductions of VEGF and VEGF receptor protein, with increased evidence of apoptosis.143 Finally, the levels of VEGF in BAL fluid in chronic cigarette smokers appears to be decreased.144 VEGF promotes endothelial cell growth,145 differentiation and survival, and accelerated restitution of endothelial integrity and function,146 and it is through this role that VEGF appears to have an important role in the development of emphysema.142,143 An animal model has suggested that apoptosis results in release of metalloproteinases, providing an additional link between apoptosis and lung destruction.147 Although these investigations are very recent, they suggest that apoptosis may be an important factor in the development of emphysema.
Distal Acinar Emphysema
This is the least common50 and the least well described form of emphysema. It has been referred to as superficial50 or mantle emphysema reflecting its subpleural location, as paraseptal48,102 or linear148 emphysema, indicating its involvement of the acinar periphery along the lobular septa, airways, and vessels, and as periacinar149,150 emphysema, involving the periphery of the acinus. Distal acinar emphysema usually occurs in associations with CLE or PAE.48,58,102 The emphysema is usually limited in extent, and is found most commonly along the anterior and posterior parts of the upper lobe, along the posterior parts of the upper lobe, and along the posterior surface of the lower lobe (Fig. 22–17). When extensive, it is usually more severe in the upper half of the lung (Fig. 22–18). There is often associated pleural fibrosis.148 The characteristic findings are of multiple contiguous, enlarged airspaces, varying from less than half a millimeter to more than 2 cm in diameter.
The syndrome of spontaneous pneumothorax in young adults most likely represents a manifestation of paraseptal emphysema.151,152 This syndrome occurs usually in the third or fourth decade, and more commonly in males153–155 who are classically tall and thin. Pneumothoraces can be multiple and can also occur on the opposite side to the identified side.153,156–158
FIGURE 22–18 Example of distal acinar emphysema, which can be recognized by the dominant involvement of the distal acinus along the lobular septa, vessels, and airways just deep to the pleura. The patient had chronic bronchitis and died of chronic airway obstruction (CAO). (From Thurlbeck WM. Chronic bronchitis and emphysema. Med Clin North Am 1973;57:651–668, with permission.)
Irregular Emphysema
Irregular emphysema may be the most common form of emphysema, and it is almost invariably associated with scarring, leading to the term scar or paracicitricial emphysema. Careful search of most lungs at autopsy discloses one or more scars, and adjacent to the scars there is usually irregular enlargement of the acinus accompanied by destructive change58 (Fig. 22–19).
The severity of irregular emphysema depends on the extent of damage to lung tissue. In some instances, multiple scars through the lung may lead to multiple foci of irregular emphysema. Examples of this include long-standing sarcoidosis and eosinophilic granuloma. Extensive irregular emphysema also occurs as a result of healing of tuberculosis or other infective granulomatous disease.
FIGURE 22–19 (A) An isolated scar in the lung with associated emphysema. (B) A photomicrograph of the lesion. (C) Old granuloma in the lung with associated emphysema.
Variants of Emphysema and Nonemphysematous Airspace Enlargement
Bullae
A bulla is as an emphysematous space more than 1 cm in diameter in the distended state.8 Bullae are quite commonly seen in chest films in which widespread emphysema can be recognized radiologically.159 Centrilobular emphysematous spaces may occasionally enlarge to form spaces more than 1 cm in diameter, but some bullae occur as isolated lesions in normal lungs, and symptoms in such instances depend on the size of the bullae and the degree of compression of adjacent normal lung.
Bullae have been classified into three types.149 Type I bullae typically occur independently of widespread associated emphysema, are subpleural in location, and are more common in the upper lobe and along the medial surface of the lung. They are characterized by a narrow neck at their junction with the underlying lung. They contain primarily air, and the walls are formed mainly by pleura and fibrous tissue. Although the bullae form large mushroom-like protrusions from the lung surface in the specimens, in life they are inverted into lung tissue and compress underlying lung. Types II and III bullae represent local exaggerations of widespread emphysema. Type II bullae occur only under the pleura, but they have a broad neck that approximates the diameter of the bulla (Fig. 22–20), whereas type III bullae occur in the substance of the lung.
FIGURE 22–20 Wedge resection for spontaneous pneumothorax in a young man. Much of the lesion involves the distal part of the acinus and is fibrotic. A projecting bulla is seen.
Blebs
Blebs are collections of air within the visceral pleura layers, and thus are a form of interstitial emphysema with air present in the connective tissue framework of the lung. This is a frequent complication in the neonatal period, especially with assisted ventilation. The presumed mechanism is rupture of alveoli situated along lobular septa and the bronchovascular sheaths, so-called marginal alveoli.160
Congenital Lobar Overinflation (Emphysema) in Infants
This is a striking clinical condition in which a lobe rapidly overinflates, usually soon after birth. The condition is common,161,162 and there is a strong male preponderance of cases; about half have congenital cardiac anomalies,161,163–165 and some have congenital abnormalities of the lung parenchyma.166 The left upper lobes are by far the most commonly affected; lower lobes are only rarely affected, but in ~10% of cases it is multilobar and occasionally bilateral.163 The affected portion expands so much that it compresses the remaining normal lung and presents a life-threatening emergency. In a small proportion of cases a fairly obvious partial obstruction to the bronchus due to mucosal flaps161,167 or vascular anomalies161,167–170 acts as a flap valve, allowing air to enter in inspiration but not to leave on expiration. However, it has been suggested that the majority of cases are due to hypoplasia of cartilage.161,168 However, other workers have suggested that, in some cases, the alveolar complement is increased above that present normally, and the individual alveoli are normal to decreased in size; this has been termed polyalveolar lobe, and may be a separate entity.171–173
Compensatory Overinflation
If a part of the lung collapses or is removed, the remaining lung can expand to fill the increased amount of space available. In humans, the exact way that this happens and the limits of the process are unknown. The remaining lung has been found to have from 116 to 140%174–176 of the predicted total lung capacity (TLC) following pneumonectomy. Because there is almost always disease in the affected lung, it is not clear what proportion each lung is contributing to TLC before surgery, but it is known that TLC continues to increase after pneumonectomy.176,177
Compensatory lung growth has been well documented in experimental animals.178 In animals, it appears that although compensatory growth is significant, and includes alveolar multiplication as well as airspace enlargement, it is not usually complete,179 and degree of restitution depends on animal maturity and species. In humans, pneumonectomy is usually performed well after alveolar multiplication has ceased; although alveolar enlargement occurs, the absolute change in linear dimensions are likely to be small.
Obstructive Overinflation
In this condition, a lung or a part of a lung overinflates because air is trapped due to obstruction of a major bronchus; the usual agents are tumor in adults and inhaled foreign bodies in children.180 Two mechanisms may be involved. In one, the obstruction in the bronchus may act as a ball valve, so that air enters on inspiration but does not leave on expiration. Alternatively, the bronchus may be completely obstructed and air may be trapped behind channels of collateral ventilation.
Obstructive overinflation differs in several ways from compensatory overinflation, although in both the lung contains too much air per unit of lung and lung tissue. Lungs with compensatory overinflation empty normally, whereas those with obstructive overinflation traps air.
The Aging Lung
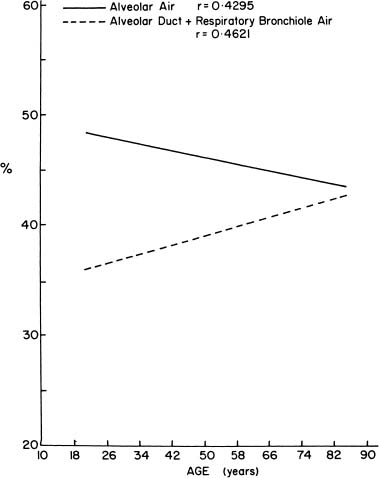
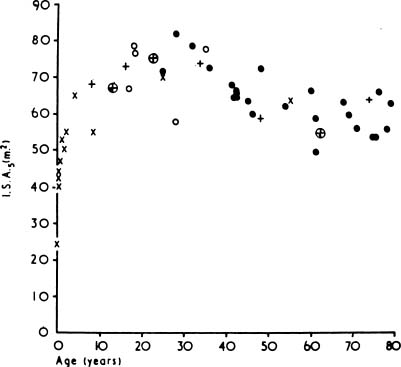
As people age, the lung “rounds out,” with increases in anteroposterior distance, height, perimeter, and area.181 Morphometric analyses have shown that there is an increase in the relative size of the alveolar ducts, a process termed ductectasia. As the alveolar ducts enlarge, the alveoli become shallower and flatter,182 and there is a relative decrease in the volume proportion of alveolar air183,184 (Fig. 22–21). This rearrangement of the geometry of the lung is accompanied by an increase in the average interalveolar distance (Lm) and a decrease in surface area (Fig. 22–22) with age. Morphologic studies suggest a loss of elastic fibers with age,185 although chemical analyses have suggested that there is no change,186 or an increase in the amount of elastin in the entire lung.187–190 Pores of Kohn are also more common in old mammals and absent in the newborn.14,15,191 Whether they increase progressively through life, thus contributing to the progressive loss of alveolar wall tissue, is unknown.192
The primary functional change in the aging lung is increased compliance with decreased elasticity;151,155,193,194 in this regard, the functional abnormalities are those of mild emphysema. The exact effect of increasing age alone on lung elasticity is unknown.
Alterations in Other Organs in Emphysema
Pulmonary Hypertension
If disease can be attributable to the effects of emphysema, it must be first distinguished from that found normally as a consequence of aging. In contrast to the progressive muscularization of the small vessels during childhood (ratio of alveoli to arteries in adults 8:1 compared with 12:1 in a 2-year-old child, and 20:1 in the newborn),195 aging is accompanied by a relative loss in the proportion of muscle with an increase in intimal fibrous tissue.196
FIGURE 22–21 When alveolar air proportion and duct air proportion in the lung are plotted against age, alveolar air proportion decreases and duct air proportion increases. (From Thurlbeck WM. Pulmonary emphysema. Am J Med Sci 1963;246:332–353, with permission.)
FIGURE 22–22 The relationship between age and alveolar surface area expressed at a standard lung volume of 5 L (ISA5). (× from Dunill, from Weibel, o from Duguid, • from Thurlbeck, ≈ from Hicken.) (From Thurlbeck WM, Wright JL. Thurlbeck’s Chronic Airflow Obstruction, 2nd ed. Hamilton: BC Decker, 1999, with permission.)
The term cor pulmonale is often applied to right ventricular hypertrophy as assessed morphologically, electrocardiographically, or radiologically, resulting from disease affecting the function and/or structure of the lung and, in the absence of congenital heart disease or disease, affecting the left side of the heart.9 Although not necessary for the diagnosis, it usually corresponds with clinical evidence of right heart failure.
Right ventricular hypertrophy (RVH) is traditionally measured by thickness of the right ventricle at autopsy. This assessment depends on the absence of ventricular dilation, and on appropriate sectioning perpendicular to the ventricular surface. A more accurate assessment can be obtained by the measurement of the weight of the right ventricle compared with that of the left ventricle plus septum197 after dissection of the ventricles from the atria at the level of the atrioventricular ring. Although the original description of the method included dissection of the left ventricle from the septum and dissection of the fat and superficial vessels, these steps are more commonly omitted for routine purposes. Using these methods, the normal weight of the right ventricle is generally less than 65 g in men and less than 50 g in women, whereas the normal ratio of left to right ventricle is between 1.9 and 4.2.198
Several studies have examined vascular structure in emphysema with or without right ventricular hypertrophy but no consistent correlations have been obtained.199–201 Cigarette smokers, with or without pulmonary hypertension, have increased muscularization of the small arterioles, an increase in muscle medial thickness, as well as intimal fibrosis in the muscular arteries.202 There appears to be a progressive shift in the numbers of smaller muscularized arteries, percent medial thickness, and percent intimal thickness of muscularized arteries from non-smokers, to smokers without obstruction, to smokers with airflow obstruction.203 Interestingly, the muscularization correlated with both the severity of emphysema and severity of small airway disease (as documented by both a subjective grading scheme and by an assessment of airway narrowing). Increases in intimal thickness with longitudinal muscle formation are a common feature in lungs of patients with airflow obstruction.201,204,205 This appears to be due to migration of muscle fibers from the circular layer rather than to in situ transformation of myofibroblasts.206
Hypoxia has often been suggested as an explanation for pulmonary hypertension in chronic airflow obstruction. The best evidence to implicate this has been the study of lungs from subjects who lived at high altitude207–210 (so-called alveolar hypoxia) who have a striking incidence of muscularization of arterioles. The rationale behind bronchiolar hypoxia is the hypothesis that ventilation/perfusion inequalities induced by emphysema and abnormalities in the small airways produce alveolar hypoventilation.211–217 However, this hypothesis has not been supported by a large clinical study.218 The degree of vascular changes is more clearly associated with severity of airflow obstruction, rather than with extent of emphysema. Finally, right ventricular hypertrophy seems to correlate best with severity of pulmonary hypertension or of airflow obstruction rather than with the presence of emphysema.
In summary, there does not appear to be a direct association between the presence of emphysema per se and right ventricular hypertrophy. It seems, however, that alterations of the structure of the vasculature can occur in emphysema, but might not be as a direct consequence of emphysema; rather, there may be an association with disease in the adjacent airway.
Pulmonary Veins
Changes in pulmonary veins have also been described in ~50% of patients with chronic airflow obstruction and right ventricular hypertrophy.219 These changes consist of medial hypertrophy and arterialization (a distinct internal and external elastic lamina of at least part of the circumference) of pulmonary veins less than 150 μm in diameter. Similar changes were seen in high-altitude residents and in a patient with the pickwickian syndrome.
Bronchial Arteries and Veins
Bronchial arteries have been found to be increased in patients with chronic airflow obstruction.220–222 Bronchial veins have also been found to be enlarged.220,222,223 The consequences of these lesions are not known.
Liver
An additional feature of α1-AT deficiency is that some (~10%) subjects with ZZ homozygotes have developed liver disease,224,225 which may progress to cirrhosis.226–231 Characteristic smooth, eosinophilic, periodic acid-Schiff (PAS)-positive, diastase-resistant, cytoplasmic inclusions are found in the parenchyma of the hepatocytes, in both cirrhotic and noncirrhotic livers, of patients with α1-AT deficiency.232,233 Immunoperoxidase studies indicate that the granules consist of α1-globulin or a closely related substance, and that the granules probably represent synthesized but unreleased α1-antitrypsin. The globules not only are found in homozygotes but also may be found in the livers of heterozygotes.77,234
Emphysema and Nutrition
Patients with severe CAO may have progressive weight loss (reviewed elsewhere235), and indeed, thin body habitus was included as part of the clinical type known as “pink puffer.” Patients who lose large amounts of weight appear to have a greater mortality than those with stable weight,236–242 and weight also appears to be an independent predictor of mortality.236,237 Weight is not, however, a contributing factor to emphysema.
Relationships of Measurements to Pulmonary Function
It is actually very difficult to determine the effect of emphysema on pulmonary function when dealing generally with a multifaceted disease state. Even when only emphysema is present (i.e., no tobacco-associated small airways bronchiolitis, such as in nonsmoking patients with α1-AT deficiency), pulmonary function tests generally reflect the state of the nonemphysematous or microscopically emphysematous parenchyma.6 This is true not only with tests of airflow such as FEV1 and FEV/FVC but also with measurements of pleural pressure.6 Nevertheless, many studies have shown correlations between tests of lung function and emphysema extent243,244 (reviewed elsewhere38).
Chronic Bronchitis
Definition
Chronic bronchitis is defined in functional terms: “Chronic bronchitis refers to the condition of subjects with chronic or recurrent excess mucus secretion in the bronchial tree.”8,245,246 No instrument exists to measure the amount of mucus secreted into the bronchial tree, and so a clinical definition of increased sputum production has been adopted in that the production of any sputum, whether expectorated or swallowed, is regarded as abnormal. Chronic is defined as “occurring on most days for at least 3 months in the year for at least two successive years.” Although not present in the definition, sputum production is, in most instances, accompanied by chronic cough.
Enlargement of the Tracheobronchial Seromucous Glands
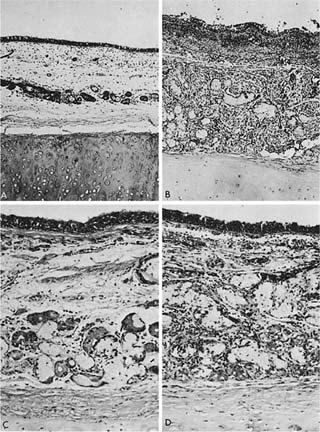
Because chronic bronchitis is defined as excess secretion of mucus, it is logical to expect that the morphologic counterpart would be excess mucus in the airways and enlargement of the mucus-secreting apparatus. Although mucus is secreted by both bronchial glands and goblet cells, the volume of the tracheobronchial seromucous glands appears to be approximately 100 times larger than the volume of the goblet cells.247 The bronchial glands lie in the submucosa and are connected to the surface by a duct through which the secretions reach the airway lumen. The glands consist of branching tubules, lined by mucous cells and darker, granular cells referred to as serous cells, both of which secrete mucosubstances. Enlargement of bronchial glands is due to both an increased number of cells (hyperplasia) in the mucous glands248 as well as a probable enlargement of the cells (hypertrophy). It is thus appropriate to refer to mucous gland enlargement (MGE). The histologic changes that occur in chronic bronchitis are illustrated in Fig. 22–23.
The Reid Index
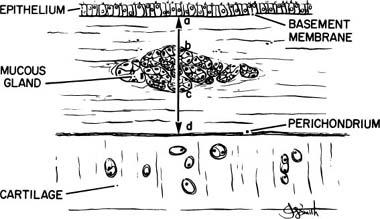
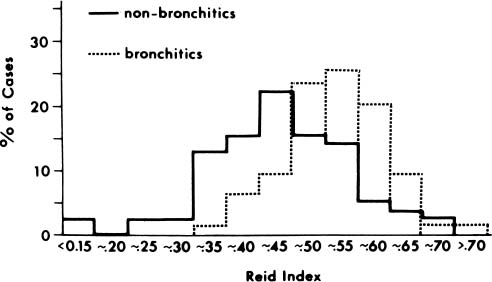
The first quantitative description of MGE in chronic bronchitis was devised by Reid,247 who measured the thickness of the bronchial glands on histologic sections of bronchi, and compared this value to the thickness of the bronchial wall, defined as the distance from the basal lamina of the epithelium to the inner perichondrium of bronchial cartilage. This ratio of bronchial gland thickness to bronchial wall thickness is now usually referred to as the Reid index of mucous gland size. As glands increase in size, so does their thickness in a cube root function relationship to the volume. The gland thickness forms the numerator of the Reid index, and thus enlargement of tracheobronchial glands causes an increase in the Reid index. Reid found that the normal index in nonbronchitic subjects was 0.26, with a range of 0.14 to 0.36. In chronic bronchitis the range was 0.41 to 0.79, with a mean value of 0.59. Reid also found that there was a close correlation between the Reid index and the daily amount of sputum. There has been ample confirmation of the increase in the Reid index that occurs in chronic bronchitis,249–253 and these studies enable one to make a reasonably precise interpretation of the index.
The exact way in which the Reid index has been, or should be, measured has not always been precisely described. An individual gland varies in thickness from place to place. In addition, the glands seen in a single section of bronchus vary in size, and many of them lie at the edges of the cartilage or between and outside the cartilage. For these reasons the Reid index is not easy to measure, and subjective judgments must be made concerning which glands should be measured and where the measurements should be made on them. It is, therefore, essential to follow a rigid system of measurement if intra- and interobserver variations are to be minimal and to ensure appropriate data for analysis.249 Specifically, it appears to be necessary to discard bronchi that are acutely inflamed or hemorrhagic, or in which the subepithelial tissue is separated from the cartilage, a relatively frequent occurrence; and only main and lobar bronchi should be utilized. The measurements should be made at the maximum thickness in glands that lie where the epithelium and cartilage are roughly parallel; glands at the ends of cartilage should not be measured. The basement membrane to inner perichondrium distance must be measured on exactly the same line (Fig. 22–24).
FIGURE 22–23 The histologic appearance of the bronchial wall of a nonbronchitic (A,C) is contrasted with that of a chronic bronchitic (B,D). (A) The tracheobronchial seromucous glands form only a small proportion of the thickness of the bronchial wall. Note the separation artifact where the bronchial mucosa has separated from the cartilage (×80). (C) The mucous and serous acini (tubules) more clearly, and scattered lymphocytes are more apparent (×150). (B,D) The glands form a much higher proportion of the wall. The bronchial submucosa is thickened by approximately the increase in thickness of the gland (B, ×80; D, ×120). (A and B from Angus.741)
The results of several studies demonstrate that the increase in the Reid index in chronic bronchitis is small,247,250,253–257 and there is considerable overlap with the data from nonbronchitics (Fig. 22–25). Although both categories have a unimodal distribution curve, when the Reid index is less than 0.36, only 6% of patients have chronic bronchitis; when it is over 0.55, 70% are chronic bronchitics. Similar results have been found by others, although the cutoffs and percentages differ.251,252,255 Fig. 22–25 also indicates the predictability of chronic bronchitis as determined from the Reid index. Predictability is good at high and low levels, but in the range of 0.36 to 0.55, when the bulk of patients are to be found, prediction is poor. Because of the gradual transition from “normal” small glands to “abnormal” large glands, there can be no sudden change in gland size between bronchitics and nonbronchitics. This poses a conflict between the morphologic concept of chronic bronchitis and the clinical one. The clinical definition of bronchitis implies an “on-off” situation, in which patients either are or are not bronchitic. Morphologically, however, the change in gland size is gradual and there is a considerable transition zone in which it is uncertain whether the glands are enlarged or not.
FIGURE 22–24 The Reid index is calculated by measuring the maximum thickness of a bronchial seromucous gland internal to the cartilage (b,c) and dividing this by the bronchial wall thickness. The latter is the distance from basement membrane to inner perichondrium (a–d). (From Thurlbeck WM. Chronic obstructive lung disease. In: Sommers SC (ed): Pathology Annual. New York: Appleton Century Crofts, 1968:367–398, with permission.)
It is also likely that a measurement of intrabronchial secretions of mucus would have a continuous distribution curve of the amount secreted. It then follows that “subclinical bronchitis” must also exist. That is, some patients may chronically hypersecrete mucus, but not sufficiently to produce sputum. Such patients might have a milder form of disability than that noted in the more typical.
Many authors have stressed that the Reid index is similar in different airways from the same subject.247,255,258–261 This is certainly true of the major airways,255,258,259 and the two main stem bronchi provide a satisfactory sample of the main and lobar bronchi.258 However, striking variations may occasionally be found.255,258–261 It has been suggested that these variations, referred to as “regional chronic bronchitis,” might result in severe abnormalities of ventilation/perfusion ratios with resultant cor pulmonale.260
An increase in mucous gland size has not been consistently found in cigarette smokers, nor have consistent differences been identified between heavy and light smokers.247,249 The Reid index (and mucous gland proportion) is larger in young children than in older children and adults,262,263 although it is unclear whether there is any further change with age.93,251,264–267 When bronchiolitis and cystic fibrosis was present in children, the mucous gland proportion was over 20%, a value seldom found in adult bronchitics.263 The Reid index was higher in children in whom atelectasis of the lung was found at postmortem examination, suggesting that mucous plugging may be an important cause of atelectasis in this age group.262 When only nonsmokers are considered there is no difference in the Reid index between men and women.266
The relationship between mucous gland hypertrophy and lung cancer is of particular interest, because it is known that chronic bronchitics have an incidence of lung cancer that is about double that of nonbronchitics even when matched for smoking habit,268 and in China, chronic bronchitis is a risk factor specifically for the adenocarcinoma histologic subtype.269 Finally, there is a very high frequency of mucous gland enlargement in lung cancer, which approaches that found in chronic bronchitis,264 even patients with lung cancer have only a 25% frequency of chronic bronchitis.
FIGURE 22–25 The Reid index in chronic bronchitics is compared with that in nonbronchitics. Note that the Reid index is shifted to the right in the chronic bronchitics. Below a Reid index of 0.35, patients are seldom bronchitic; above 0.55, patients are usually chronic bronchitics. However, in the intervening range, where most values are found, the Reid index is poorly predictive of the presence of chronic bronchitis. (From Thurlbeck WM. Aspects of chronic airflow obstruction. Chest 1977;72:341–349, with permission.)
Only one group of observers has examined the effect of environment, and they were unable to show a difference when they compared the Reid index and number of acini per unit area in urban and rural subjects.264 Although the Reid index values in Jamaica266 are lower than those reported by any others, it is unclear whether this observation is due to the environment, differences in technique, or racial differences.
Other Measurements of Gland Size
Mucous Gland Proportion
It is possible to assess the proportions of the bronchi formed by various constituents, such as cartilage or mucous glands, by using the point-counting technique.251,260,261,267,270–273 The method has many apparent advantages: it is possible to measure all mucous glands, rather than only those internal to the cartilage, and arbitrary points are not selected on the individual glands; rather, the total gland proportion is measured, thus avoiding arbitrary decisions about which glands to select and where they should be measured. Also, the common artifact of separation of the mucous membrane from cartilage is not a major problem, because the space can be included as a lumen and thus ignored as a bronchial wall component. One clear-cut advantage of the point-counting method is that it provides information concerning the proportion of bronchial cartilage and muscle as well as mucous glands. However, this method is time-consuming, and it may be difficult to determine the outer margins of the bronchial wall, particularly in intrapulmonary bronchi, thus altering the total number of hits on the bronchial wall and changing the proportions. Using this method nonbronchitics had a range of mucous gland proportion of 7.6 to 16.7%, with a mean of 12.7%, whereas the mean value found in the bronchitics was 27.8%.270 Once again, however, there is considerable variation among authors.251,256,271,274–276
The mucous gland proportion has been found to be closely related to the Reid index, although such correlations were not found when severe airflow obstruction was present.277 Some argument exists as to whether mucous gland proportion has a greater correlation with chronic bronchitis than the Reid index.271,272,278 Nevertheless, a significant correlation between the volume proportion of glands and daily sputum volume279 has been identified, and a stronger correlation was found between the volume proportion of glands and the amount of sputum produced than between the Reid index and amount of sputum.277
Measurement of Absolute Gland Size
Absolute measurements of glands can be made, the most common of which are gland thickness249,250 and gland area.275,276,280 Measurements of the acinar size in the individual glands247,249,264,271,281,282 have also been performed. The absolute area of glands diminishes progressively distally in the bronchial tree,275,276,280 requiring normalization for airway size, and absolute size is affected by shrinkage of sections and by obliquity of the section through the glands. It is also possible that mucous glands may vary with the size of the individual, because most organs do. There is a poor correlation between the Reid index and absolute size as measured in histologic sections,272 and although the sensitivity of these measurements has not been thoroughly tested, one study found that gland thickness discriminated less well than the Reid index between groups of bronchitics and nonbronchitics.250
There is no question that the acini increase in size in chronic bronchitis.247,249,255,264,271,282,283 A significant correlation of +0.79 has been reported between the average acinar diameter and the Reid index,271 but the association between the number of acini per unit area and the Reid index has not been strong.249,255,284 Like the Reid index, however, acinar diameter has a unimodal frequency distribution. Nonbronchitics were found to have a mean value of 41.6 μm with a range of 33.5 to 49.8 μm,283 whereas in chronic bronchitis the acini had a mean diameter of 49.3 μm with a range of 40 to 56.1 μm.283 Nonetheless, the size of acini is less powerful than the Reid index in distinguishing between groups of bronchitics and nonbronchitics.249,264,283
Other Airway Changes
Ratio of Mucous to Serous Acini
There are two types of secretory cells in the mucous glands: mucous and serous. Subjective descriptions suggest that there is an increased proportion of mucus-secreting cells in the tracheobronchial glands in chronic bronchitis.247,285–287 Although detailed point counting was able to demonstrate a slight shift toward increased proportion of mucous cells, this did not reach statistical significance. In addition, the proportion of mucous cells correlated poorly with both the Reid index and the mucous gland proportion,271,283 suggesting that the relative proportions of mucous cells were not a sensitive indicator of chronic bronchitis.
Goblet Cell Metaplasia
Mucus is also secreted by the goblet cells; although these cells have a much smaller volume than bronchial glands, this does not necessarily mean that much less mucus is secreted from goblet cells.247 Further, although goblet cells may not produce large amounts of mucus, their presence in bronchioles may be critical in production of small airway dysfunction. Bronchial glands do not extend to these small airways; if bronchioles are obstructed by mucus, the mucus must come from goblet cells or there must be retrograde movement of mucus from the central airways.
Studies of goblet cells in the larger airways have not consistently shown an increased number in chronic bronchitis.247,256,257,259,274,279 No relationship between the Reid index or volume proportion of glands and goblet cell metaplasia has been found,288 and goblet cell metaplasia is not significantly more common in lungs in which the Reid index was increased.289
Mucus in the Airways
One would anticipate that there should be excess mucus in the airways of patients with chronic bronchitis, but the extent of mucus and its distribution within the tracheobronchial tree in nonbronchitics and bronchitics are not certain. The normal nonbronchitic, nonemphysematous lung contains only ~1 mL of mucus in the peripheral airways of the lung, or ~0.02% of the lung volume. Two studies have found increases in airway mucus in chronic bronchitis, although the results vary quite markedly in magnitude.279,290 Examination of their results suggests that when severe airflow obstruction is present, there is a greater amount of mucus in the central (between eight-and 10-fold over controls) and peripheral airways than is present when isolated chronic bronchitis exists (approximately a 3.5-fold increase over controls).
Inflammation of Bronchi
Evidence of Inflammation
Surprisingly, inflammatory cell infiltration has not, until recently, been emphasized as a histologic feature of the bronchi in chronic bronchitis, nor has it been well studied. Examination of sputum and BAL fluid has shown abundant evidence of inflammation291 with increased numbers of neutrophils in smokers,292–294 and more specifically in chronic bronchitics.295,296 In addition, an increased amount of neutrophil derived peroxidase accompanied the neutrophils, indicating their activation.296
In pathologic descriptions of chronic bronchitis (usually to differentiate between asthma and chronic bronchitis) evidence of inflammation is consistently reported,270,287,297–299 with the participating cells predominantly mononuclear, but with some neutrophils300 and the occasional eosinophil. The bronchial glands of smokers with bronchitis have increased populations of neutrophils and macrophages, with a decreased CD4/CD8 T-cell lymphocyte ratio.301 In general, there is no association between the severity of the inflammatory infiltrate and the Reid index or other measurements of gland enlargement.259,288,302,303
To summarize a large number of studies,248,274,296,304–311 it is apparent that in cigarette smokers without bronchitis inflammation is about equal in all cartilaginous airways, whereas in smokers with bronchitis inflammation is greater in airways with a diameter or 4 mm or more. T cells are normally present in airways of nonsmokers, and smokers without bronchitis, whereas B cells are extremely scanty or absent; in patients with chronic bronchitis compared with both control nonsmokers and nonsmoking bronchitics, the total number of epithelial T (CD3) cells as well as helper T (CD4) and suppressor (CD8) cells appear increased. Chronic bronchitics may have a higher percent of neutrophils in their BAL fluid, and those with more than 20% neutrophils have lower values for diffusion capacity for carbon monoxide (DLCO), FEV1, FEV1/FVC, and forced expiratory flow after 25 to 75% of vital capacity has been expelled (FEF25–75) than those with a lesser percentage. The nature and severity of the inflammatory cell infiltrate may not change in long-term (1 to 18 years, mean 13 years) ex-smokers whose bronchitis persists.
There are very limited data regarding alterations of cytokine profile in chronic bronchitis. Sputum tumor necrosis factor-α (TNF-α), interleukin-8 (IL-8), and IL-4 have been reported to be increased in the sputum, and IL-4 and IL-5 in the bronchial glands and subepithelium of chronic bronchitics, whereas IL-4, IL-5, and regulated-upon activation in normal T cells expressed and secreted (RANTES) appear to be increased during exacerbations.312–315
Eosinophilia in Chronic Bronchitis
Eosinophils are often found in the sputum,316–319 BAL fluid,248 and lung parenchyma248,287,297–299,312,320 of patients with chronic bronchitis. The numbers, however, vary markedly, ranging from 0 to 2.75%.287,297 A significant overlap between eosinophil counts in the bronchial walls in nonfatal asthmatics, normals, and chronic bronchitics has been reported.297,299 Eosinophils appear to be especially prominent during exacerbations.321
Other Bronchial Lesions
Pits in the Bronchial Wall
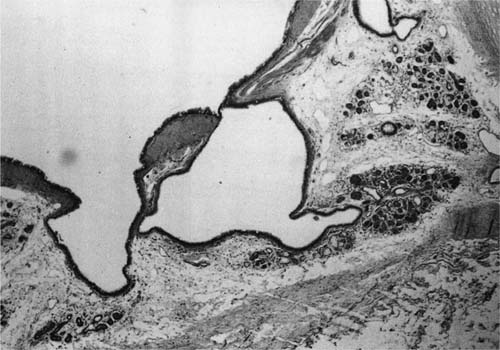
The openings of the tracheal-bronchial glands into the lumen can be seen using a hand lens or a dissecting microscope (Fig. 22–26). The ducts may also be distended with mucus and the mucus may protrude into the lumen of the bronchus and be visible grossly. Radiologically, they appear as small diverticulum-like structures best seen along the inferior margins of the lobar bronchi,322,323 and most commonly found along the margins of the cartilaginous rings.323 Histologically, these are well-defined small pits that are dilated openings of mucous glands or larger pits into which two or more ducts of mucous glands open (Fig. 22–27). The suggested sequence of formation starts with dilatation of gland ducts, depressions in the epithelium, and finally herniation of the bronchial epithelium through the muscular layer, which in itself may also be abnormal.324,325 Whether these lesions are pathognomonic of chronic bronchitis,322,323 or perhaps of mucous gland hyperplasia, is uncertain because these abnormalities have been identified in patients without chronic bronchitis,324,326 and are commonly found in patients with bronchiectasis.
FIGURE 22–26 Longitudinally transected bronchus from a chronic bronchitic. Note pit in bronchial wall (between arrowheads) and mucus being extruded from glands, just to the right of the pit (×8 approximately).
Oncocytes
Oncocytes are defined as large cells with granular, deeply eosinophilic cytoplasm and often with small dark nuclei. They are sometimes strikingly prominent in the bronchial mucous glands of chronic bronchitics. It is unclear whether they are more frequent in the bronchial glands in patients with chronic bronchitics than in nonbronchitics, or whether they are only indicative of an age effect.327
Muscle
The status of bronchial smooth muscle in chronic bronchitis is controversial, with some studies finding that there was no significant increase in the average volume proportion of bronchial muscle in patients with chronic bronchitis,270,274 others demonstrating an increased proportion,328 and still others finding differences when moderate or severe chronic airflow obstruction was present.288 It has also been suggested that the increase in muscle is not generalized but occurs most obviously in the left lower lobe bronchus.328 Measurements of the absolute area of airway smooth muscle on histologic sections from patients with chronic bronchitis compared with similar bronchi from nonbronchitics have shown no increase in tracheal smooth muscle in chronic bronchitis, but more than double the muscle area in the bronchus to the basal segments and to the posterior basal segmental bronchus to the left lower lobe.329,330
The increase in muscle mass in chronic bronchitis is due to hyperplasia of muscle cells rather than to hypertrophy.329,331 The significance of muscle increase is not apparent, because no relationship has been shown between muscle and fluctuations in flow rates or reversibility to bronchodilator in one study332 or with methacholine sensitivity in another.333
Airway Wall
One of the striking changes in bronchitics is increased vascularity of the subepithelial tissue, referred to as “capillary proliferation,” which is present in a minority of the large airways in chronic bronchitics.297 This finding, together with associated edema and mucous gland enlargement, might result in airway wall thickening, bronchial narrowing, and flow obstruction.334,335 It is not clear whether all airways are involved.253 It is also not clear as to whether these changes are related to the presence of chronic bronchitis, or whether they are generic changes secondary to cigarette smoke, and are related to airflow obstruction (see later in this chapter).
FIGURE 22–27 Pit in bronchial wall formed by two dilated bronchial mucous gland ducts (H&E, ×40 approximately).
Cartilage and Bronchial Wall Atrophy
Early studies described cartilaginous atrophy and thinning of the bronchial wall in the segmental and subsegmental airways of patients with chronic bronchitis and emphysema, although the authors believed that the changes correlated better with emphysema than with bronchitis.336 Several groups of observers have been unable to show significant alterations in the area of cartilage in patients with chronic bronchitis,270,276,283 or in lungs with high Reid indices,337 although decreased cartilage was also identified in emphysematous lungs.337
When bronchi were dissected, opened longitudinally, and measured for extension of cartilage,338 it was found that circumferentially arranged cartilage extended farther distally in nonbronchitics than in bronchitis with a striking negative correlation between the Reid index and this distance (r = −0.85). In addition, there was a deficiency of cartilage in segmental specimens from several chronic bronchitics.
Histologic signs of cartilage degeneration, as judged by loss of cellular or pericellular metachromasia and vacuolated or empty lacunae, were found in subjects with chronic bronchitis, asthma, or emphysema, although the degree of change was most marked in chronic bronchitis.339 Interestingly, the degree of degeneration correlated with the amount of perichondral fibrosis in addition to the percentage of neutrophils in the bronchial wall.
Small Airway Disease (Bronchiolitis)
Extensive lesions in bronchioles have been described in patients dying of symptomatic chronic airflow obstruction. It should be pointed out that these small airway lesions are not necessarily a complication of, or due to, chronic bronchitis. It is possible, for example, that cigarette smoke has a direct effect on central airways, producing hypersecretion of mucus and thus chronic bronchitis. In the peripheral airways, its action might be different, producing bronchiolitis directly or indirectly, by predisposing to viral or bacterial infection. Thus, if cigarette smoking was the most important cause of both conditions, one might expect them to occur together more frequently than chance would allow, but they could occur independently and would not be etiologically or pathogenetically interrelated. This was emphasized in a study examining patients with severe airflow obstruction, in which there was no relationship between the Reid index and bronchiolitis.288
Epidemiology of Chronic Bronchitis
Extensive surveys have been made of the incidence of chronic bronchitis using a standard questionnaire.340–370 The validity and repeatability of the assessment of respiratory symptoms and of the questionnaire have been examined371–373 and, in general, different observers obtain the same information, patients provide similar information on repeated questioning, and population groups produce similar results even after a lapse of some years.357 Chronic bronchitis is common, affecting about 10 to 25% of the adult population. Bronchitis is more common in men than in women and more common after the age of 40 years than before. Smoking, cigarette smoking in particular, is the most important factor associated with bronchitis. Other variables are less obvious, but after adjustment for smoking it is likely that women are less susceptible than men, that country dwellers have a lower incidence than city dwellers, that workers in some dusty occupations have a high incidence of bronchitis, and that chronic bronchitis is more common in Britain than in the United States.
Death certificates have shown remarkable differences374 in the frequency with which chronic bronchitis has been considered to be the cause of death in different countries. Undoubtedly differences in terminology among countries are sufficiently great to question the validity of such comparisons, and it should be reemphasized that specific diagnostic terms such as “chronic bronchitis” or “emphysema” have little meaning on death certificates or on discharge diagnoses other than indicating the probably presence of chronic airflow obstruction. Nevertheless, existing data demonstrate the coexistence of chronic bronchitis and emphysema.
Etiology and Pathogenesis of Chronic Bronchitis
Knowledge of the etiology of chronic bronchitis is derived from epidemiologic studies. Typical studies have shown a frequency of chronic bronchitis of ~5% in nonsmokers, rising to ~20% in light smokers (1 to 14 cigarettes per day), and 30% in smokers of 15 or more cigarettes per day.340,345,375,376 In these same series, the frequency of chronic bronchitis was almost doubled in industrial workers. These studies suggest additional environmental causes of bronchial inflammation.
The older literature emphasized the rule of bacterial and viral infection in the pathogenesis of chronic bronchitis.377–396 However, it seems unlikely that bacterial infection is important in initiating chronic bronchitis, but it is probably significant in maintaining it and may be critical in producing exacerbations, which form such a characteristic part of chronic bronchitis.
Although several early studies had noted that goblet cell metaplasia could be produced by instillation of neutrophil elastase,397–400 with the advent of modern molecular biologic techniques, the relationship between mucin gene (predominately MUC5AC) upregulation by neutrophil elastase, TNF-α, and oxidants has been greatly clarified. The epidermal growth factor receptor appears to be integral to the development of goblet cell metaplasia, and can be activated through ligand-dependent or ligand-independent means.401–412 The inflammatory mediators IL-8 and IL-13 are involved in goblet cell differentiation, and have interesting feedback loops to TNF-α in addition to autocrine relationships with the epidermal growth factor receptor.413–415
Relationships of Measurements to Pulmonary Function
The early, classic study of Fletcher and Peto416 found that the presence of mucus hypersecretion did not result in significant airflow obstruction. However, there may be an association of chronic bronchitis with an accelerated decline of pulmonary function,417 presumably through involvement with exacerbations.6
Tobacco Associated Bronchiolitis (Small Airway Disease, Chronic Obstructive Bronchiolitis)
Bronchiolitis Versus Small Airway Disease
By definition, the term bronchiolitis would mean any condition characterized by inflammation of the bronchioles, and many of these conditions are discussed in their respective chapters. Although tobacco-induced bronchiolitis is by far the commonest and most important form of bronchiolitis, it is not usually the first entity considered when one hears the term. Rather, the terms small airway disease, tobacco-associated bronchiolitis, and chronic obstructive bronchiolitis connote the (generally) inflammatory alterations (Fig. 22–28) found in the bronchioles in people who smoke cigarettes.
Concept of Small Airway Disease
The concept of small airway disease was introduced in the late 1960s when, using retrograde airway resistance measurements, it was found that distending pressures did not have an effect on lung resistance, suggesting that it was the airways rather than the emphysematous lung destruction that was responsible for the resistance increase (up to 80% of total resistance) in the emphysematous lung.418 This was supported by a study that showed that pulmonary conductance (the inverse of resistance) correlated with mean bronchiolar diameter.419 Calculations of the cross-sectional area of the small airways (~239 cm2) would suggest that the airways contributed very little to total airflow resistance in the normal state, and it was considered that significant alteration of the airways would be necessary before standard tests of airflow would become abnormal. The small airways were thus considered the “quiet” or “silent” zone420,421 of the lung.
FIGURE 22–28 Inflammatory alterations of the membranous (A–C) and respiratory bronchioles (D–F). (A) Intraluminal mucus accumulation in a membranous bronchiole with pronounced goblet cell metaplasia. (B) Fibrosis of the airway wall and luminal distortion in a membranous bronchiole. Note also the pigment accumulation in the airway wall. (C) Inflammation in the wall of a membranous bronchiole, which also demonstrates thickening of the airway smooth muscle. (D) Inflammation in the lumen of a respiratory bronchiole. (E) Inflammation in the wall of a respiratory bronchiole associated with distortion of the airway lumen. (F) Fibrosis of the wall of a respiratory bronchiole. (From Wright JL, Cosio MG, Wiggs BR, Hogg JC. A morphologic grading scheme for membranous and respiratory bronchioles. Arch Pathol Lab Med 1985;109: 163–165, with permission.)
These physiologic data gave impetus to the development of tests that were thought to be able to detect alterations in the small airways (nitrogen washout, helium, FEF25–75).422 Thus the concept encompassed by the term small airway disease was that the structural alterations of the membranous and respiratory bronchioles, by themselves and in the absence of emphysema, could increase airway resistance and cause airflow abnormalities, which were of sufficient magnitude to produce clinical airflow obstruction, and which could be detected by physiologic tests. The concept suggested that if the lesions in the small airways occurred in a temporal fashion, and if they could be detected using the recently developed tests, and the patient could be convinced to stop smoking, then severe clinical CAO could be prevented. The fallacy of this argument is discussed later, and it is my opinion that the term small airway disease has outlived its usefulness and should be replaced with the term smokers’ bronchiolitis or tobacco-associated bronchiolitis.
Smokers’ (Tobacco-Associated) Bronchiolitis
Abnormal small airways have been consistently recognized in patients with mild and severe chronic obstructive limitation (discussed elsewhere38). Because Poiseuille’s law would indicate that the resistance to airflow varies inversely with the fourth power of the airway radius, the effective radius could be decreased in several ways: (1) the airway lumen contained an increased amount of mucus or inflammatory exudate; (2) the lumen itself could be narrowed and made tortuous by the presence of granulation tissue plugs or their fibrotic residua; (3) the lumen could be narrowed because the subepithelial (submucosa) portion of the airway has increased in size with inflammatory cells and fluid, or fibrous tissue; (4) the airway lumen could become narrowed and distorted if it lost its supporting (tethering) alveoli. All of these possibilities appear to be applicable to the small airways in cigarette smokers.
Analysis of Structure
Bronchiolar Narrowing
The usual method to assess airway size is the internal bronchiolar diameter, with the measurements taken on cross section or on the short axis of the ellipse.423 If direct cross sections, rather than elliptical sections, are obtained, the internal perimeter of the basement membrane can be used as an index of size.424 The data collected by these measurements can be expressed as a mean or median value, by construction of histograms,423,425–429 or as cumulative distribution curves (Q plots).428 Alternatively, this value can be compared with the size of the adjacent pulmonary artery; the ratio should be roughly equal to 1.430
Using this methodology, it has been shown that the airways of current and ex-smokers are thickened, or have decreased internal diameter, or have a shift in size distribution toward the smaller sizes (reviewed elsewhere38). Similar findings are present when the lungs with at least moderate emphysema or with decreased airflow are examined.428,431 It appears, therefore, that some portion, although certainly not all, of the decreased airflow found by individual pulmonary function tests can be attributed to narrowing of the membranous and respiratory bronchioles.
Conformity/Deformity Index
This index is based on the concept that airways approximate cylinders, and that sections through the cylinders would therefore be elliptical.288,426,432 Deviations from the mathematical calculations of theoretical ellipses would then be indicative of airways distortion, providing a numerical equivalent to the descriptive date present in the early literature.433–439 Using this index, it has been found that the lungs with significant emphysema had irregular and distorted airways.288,426,432 The changes may be due to airways alteration, or may be secondary to emphysematous loss of support, or some combination of both.
Three-Dimensional Reconstruction
Bronchial casts have been used to examine the overall structure of the airways, and have demonstrated that lungs from chronic cigarette smokers had widespread airway stenoses of variable length with either an eccentric or concentric arrangement. Actual reconstruction of airways from serial sections is time-consuming and tedious, and therefore is limited to only a few airways.440 It is, however, a superb method for visualizing the areas of stenosis, dilatation, and tortuosity, and the rough and irregular airway lumina in emphysematous lungs.426,440
Grading Methodologies
The pathology grading systems were devised as a method by which a relatively quick and simple analysis of a variety of individual pathologic processes could be done; some processes, such as pigment deposition, are extremely difficult to quantify. Early grading methods were a simple present/absent analysis,441,442 with modifications to indicate percentage of airways involved. Neither of these two methods was useful for assessing the severity of the individual lesions. The most frequently used scheme443,444 involved assigning each airway a separate score (0 to 3) for a variety of parameters, based on comparison with a series of photographed standards. To provide an estimation of overall severity for each parameter, all of the airway scores are added, and then expressed as a percentage of maximum possible score (number of airways times 3). Summing the scores for all parameters provided a total pathologic score. On average, a single lobe of lung is relatively representative of the lung as a whole.445–448 Grading analysis has allowed investigators the ability to extend their structural analyses and ascertain, but only in a general, rather than a detailed numeric, fashion, what abnormalities are responsible for increased airway wall thickness,445,449 and how these morphologic findings relate to airflow obstruction.428,449,450
Probably the most valuable contribution was that demonstrating that young smokers characteristically develop collections of macrophages in the lumina of the respiratory bronchioles.441 Other studies have shown that the membranous and respiratory bronchioles in cigarette smokers have an increase in mural and luminal inflammation, accompanied by an increased amount of fibrous tissue as well as goblet cell metaplasia.30,445,451,452
Alveolar Attachments
Analysis of the alveolar attachments involves examining the alveolar that are adjacent to the adventitia of the airway wall and determining whether these alveoli are intact or have lost their integrity (Fig. 22–29); data are examined relative to airway size. Loss of the alveolar attachments was noted in the early descriptions of emphysema,434 but it took several years before the idea arose that radial bronchiolar support was necessary to prevent early airway closure,27,28 and loss of the support might partly explain airflow obstruction in patients with emphysema. The various investigations have demonstrated a decreased number of attachments in emphysema29,32,34,36,453 or in smokers compared with nonsmokers,30,31 and also showed correlations between the loss of attachments and airway disease.30,34,35 There are only a few studies examining correlations between pulmonary function tests and alveolar attachments,34,36,37 but all found correlations of alveolar attachment disruption with FEV1 and the nitrogen washout test, indicating that the function tests deteriorated in consort with an increase in interalveolar distance or a decreased number of alveolar attachments adjusted for airway circumference. In addition, significant correlations between alveolar attachment and percent predicted that K (calculated from pressure volume curves) as well as static recoil pressure were found, indicating that elastic recoil decreased and K increased with decreasing numbers of alveolar attachments.36
FIGURE 22–29 The pattern of alveolar attachments to the wall of membranous bronchioles in a nonemphysematous lung (top) is compared with an emphysematous lung (bottom). Note the loss of regularity of alveolar wall attachments and their sparsity. (From Linhartova A, Anderson AE, Foraker AG. Affixment arrangements of peribronchiolar alveoli in normal and emphysematous lungs. Arch Pathol Lab Med 1982; 106:499–502, with permission.)
It appears from these studies that peribronchiolar alveolar attachment destruction is related to cigarette smoking as an entity separate from emphysema, but destruction is more marked in lungs with severe emphysema. Pulmonary function may be influenced by alveolar attachment destruction in a fashion separate from the influence of emphysema.
Image Analysis
Several investigators have utilized image analysis or morphometric techniques to try to quantify the individual components of the airway. They have shown that the airways in emphysema, or airflow obstruction regardless of the presence of emphysema, have variably thickened walls with narrowed lumina.29,428,431,454
Other workers have tried to document the numbers and types of inflammatory cells in the airways. Smokers and ex-smokers appear to have increased numbers of neutrophils and mononuclear cells in the airway walls;31,455 CD8-positive lymphocytes were increased in subjects with airflow obstruction,450,456–459 and appeared to be proportional to the amount smoked. Numbers of inflammatory cell types also correlated with the severity of airflow obstruction.455,459
Relationships of Measurements to Pulmonary Function
The results of correlations must be interpreted with caution and with full knowledge of all of the pitfalls inherent in this process. It is difficult to differentiate the effects of a single lesion when many lesions can be, and are, produced secondary to chronic smoking. Thus, it may be difficult to differentiate the effects of emphysema from those of smokers bronchiolitis, and equally difficult to differentiate among the effects of the individual pathologic parameters composing smokers bronchiolitis. Furthermore, the pathologic lesions induced by cigarette smoke may or may not be not be produced synchronously, and like CAE, may not be distributed equally throughout the lung.288,460 An additional source of variance might be the type of specimen used to provide the correlation, and the length of time between pulmonary function testing and specimen receipt.
Physiologic Correlations
Many studies have found correlations between either structural or grading variables with function tests (reviewed elsewhere38). A wide variety of correlations have been found, but in general it can be seen that narrowing of the airways and inflammation and fibrosis of the airways appear to be common pathologic correlates with the individual physiologic tests. The obvious question is whether, other than answering a pathophysiologic problem, the tests actually mean anything to the individual patient and their physician.
The Fallacy of the Concept of “Early”
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree