Chapter 36 Diseases of the Breast
Anatomy
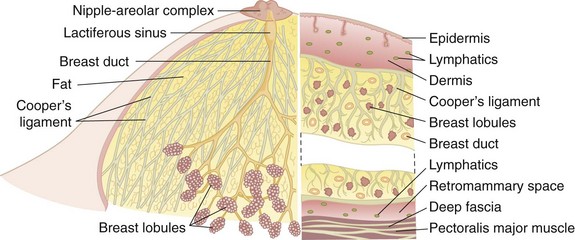
The breast lies between the subdermal layer of adipose tissue and the superficial pectoral fascia (Fig. 36-1). The breast parenchyma is composed of lobes that in turn are comprised of multiple lobules. There are fibrous bands that provide structural support and insert perpendicularly into the dermis, termed the suspensory ligaments of Cooper. Between the breast and pectoralis major muscle lies the retromammary space, a thin layer of loose areolar tissue that contains lymphatics and small vessels.
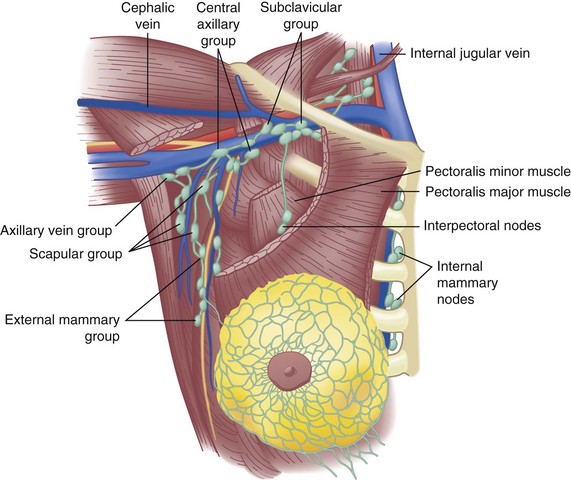
Located deep to the pectoralis major muscle, the pectoralis minor muscle is enclosed in the clavipectoral fascia, which extends laterally to fuse with the axillary fascia. The axillary lymph nodes, grouped as shown in Figure 36-2, are found within the loose areolar fat of the axilla; the number of lymph nodes is variable, depending on the size of the patient. The number of lymph nodes recovered from pathologic examination of Halsted-type radical mastectomy specimens is approximately 50 nodes.
Lymphatic channels are abundant in the breast parenchyma and dermis. Specialized lymphatic channels collect under the nipple and areola and form Sappey’s plexus, named for the anatomist who described them in 1885. Lymph flows from the skin to the subareolar plexus and then into the interlobular lymphatics of the breast parenchyma. Appreciation of lymphatic flow is important for performing successful sentinel lymph node surgery (see later). Of lymphatic flow from the breast, 75% is directed into the axillary lymph nodes. A minor amount goes through the pectoralis muscle and into more medial lymph node groups, as shown in Figure 36-2. Lymphatic drainage also occurs through the internal mammary lymph nodes as the predominant drainage in up to 5% of patients and as a secondary route in combination with axillary drainage in approximately 20%. A major route of breast cancer metastasis is through lymphatic channels; the regional spread of cancer is important to understand to provide optimal locoregional control of the disease.
Microscopic Anatomy
The glandular apparatus of the breast is composed of a branching system of ducts, roughly organized in a radial pattern spreading outward and downward from the nipple-areolar complex (see Fig. 36-1). It is possible to cannulate individual ducts and visualize the lactiferous ducts with contrast agents. Figure 36-3 demonstrates the arborization of branching ducts, which end in terminal lobules. The contrast dye opacifies only a single ductal system and does not enter adjacent and intertwined branches from functionally independent ductal branches. Each major duct has a dilated portion (lactiferous sinus) below the nipple-areolar complex. These ducts converge through a constricted orifice into the ampulla of the nipple.
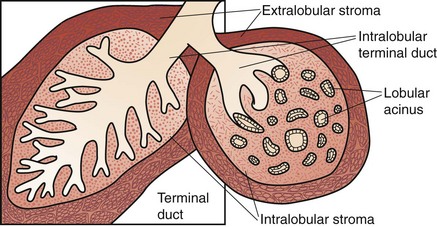
Each of the major ducts has progressive generations of branching and ultimately ends in the terminal ductules or acini (Fig. 36-4). These acini are the milk-forming glands of the lactating breast and, together with their small efferent ducts or ductules, are known as lobular units or lobules. As shown in Figure 36-4, the terminal ductules are invested in a specialized loose connective tissue that contains capillaries, lymphocytes, and other migratory mononuclear cells. This intralobular stroma is clearly distinguished from the denser and less cellular interlobular stroma and from the adipose tissue within the breast.
The entire ductal system is lined by epithelial cells, which are surrounded by specialized myoepithelial cells that have contractile properties and serve to propel milk formed in the lobules toward the nipple. Outside the epithelial and myoepithelial layers, the ducts of the breast are surrounded by a continuous basement membrane containing laminin, type IV collagen, and proteoglycans. The basement membrane layer is an important boundary in differentiating in situ from invasive breast cancer. Continuity of this layer is maintained in ductal carcinoma in situ (DCIS), also termed noninvasive breast cancer (see later, “Pathology”). Invasive breast cancer is defined by penetration of the basement membrane by malignant cells invading the stroma.
Breast Development And Physiology
Normal Development and Physiology
Prior to puberty, the breast is composed primarily of dense fibrous stroma and scattered ducts lined with epithelium. In the United States, puberty, as measured by breast development and the growth of pubic hair, begins between the ages of 9 and 12 years, and menarche (onset of menstrual cycles) begins at approximately 12 to 13 years of age. These events are initiated by low-amplitude pulses of pituitary gonadotropins, which raise serum estradiol concentrations. In the breast, this hormone-dependent maturation (thelarche) entails increased deposition of fat, the formation of new ducts by branching and elongation, and the first appearance of lobular units. This process of growth and cell division is under the control of estrogen, progesterone, adrenal hormones, pituitary hormones, and the trophic effects of insulin and thyroid hormone. There is evidence that local growth factor networks are also important. The exact timing of these events and the coordinated development of both breast buds may vary from the average in individual patients. The term prepubertal gynecomastia refers to symmetrical enlargement and projection of the breast bud in a young girl before the average age of 12 years, unaccompanied by the other changes of puberty. This process, which may be unilateral, should not be confused with neoplastic growth and is not an indication for biopsy.1
Fibrocystic Changes and Breast Pain
The condition previously referred to as fibrocystic disease represents a spectrum of clinical, mammographic, and histologic findings and is common during the fourth and fifth decades of life, generally lasting until menopause. An exaggerated response of breast stroma and epithelium to a variety of circulating and locally produced hormones and growth factors is frequently characterized by the constellation of breast pain, tenderness, and nodularity. Symptomatically, the condition is manifested as premenstrual cyclic mastalgia, with pain and tenderness to touch. This can be worrisome to many women; however, breast pain is not usually a symptom of breast cancer. Haagensen1 has recorded the symptoms of women with breast carcinoma and found pain as an unprompted symptom in 5.4% of patients. In women with breast pain and an associated palpable mass, the presence of the mass is the focus of evaluation and treatment. Normal ovarian hormonal influences on breast glandular elements frequently produce cyclic mastalgia, generally pain in phase with the menstrual cycle. Noncyclic mastalgia is more likely idiopathic and difficult to treat. Women 30 years and older with noncyclic mastalgia should undergo breast imaging with mammography in addition to a physical examination. If examination reveals a mass, this should become the focus of subsequent evaluation. Occasionally, a simple cyst may cause noncyclic breast pain, and aspiration of the cyst will usually resolve the pain. Most patients with simple cysts do not require any further evaluation unless it is a complex cyst with solid intracystic components.
Abnormal Development and Physiology
Nipple Discharge
It is important to establish whether the discharge comes from one breast or from both breasts, whether it comes from multiple duct orifices or from just one, and whether the discharge is grossly bloody or contains blood. A milky discharge from both breasts is termed galactorrhea. In the absence of lactation or a history of recent lactation, galactorrhea may be associated with increased production of prolactin. Radioimmunoassay for serum prolactin is diagnostic. However, true galactorrhea is rare and is diagnosed only when the discharge is milky (contains lactose, fat, and milk-specific proteins). Unilateral discharge coming from one duct orifice is often treated surgically when there is a significant amount of discharge (Fig. 36-5). However, the underlying cause is rarely a breast malignancy.
Galactocele
A galactocele is a milk-filled cyst that is round, well circumscribed, and easily movable within the breast. It generally occurs after the cessation of lactation or when feeding frequency has curtailed significantly. Haagensen1 has reported that galactoceles may occur up to 6 to 10 months after breastfeeding has ceased. The pathogenesis of galactocele is unknown, but it is thought that inspissated milk within ducts is responsible. The tumor is usually located in the central portion of the breast or under the nipple. Needle aspiration produces thick creamy material that may be tinged dark green or brown. Although it appears purulent, the fluid is sterile. Treatment is needle aspiration, and withdrawal of thick milky secretion confirms the diagnosis; surgery is reserved for cysts that cannot be aspirated or those that become infected.
Diagnosis Of Breast Disease
Physical Examination
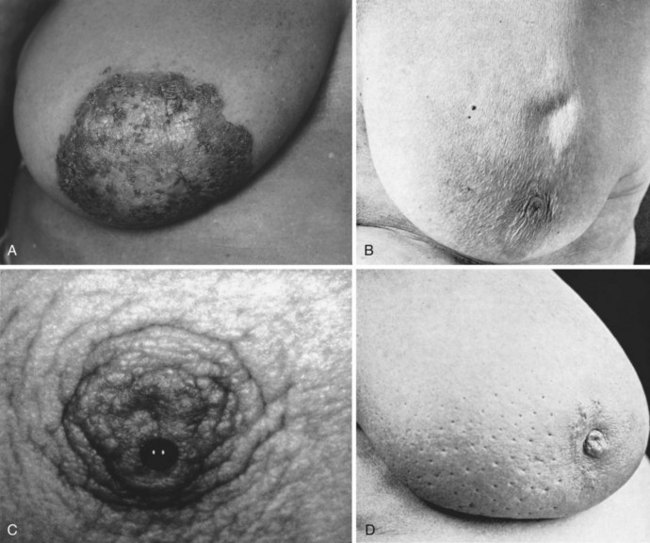
The examination begins with the patient in the upright sitting position with careful visual inspection for obvious masses, asymmetries, and skin changes. The nipples are inspected and compared for the presence of retraction, nipple inversion, or excoriation of the superficial epidermis such as that seen with Paget’s disease (see Fig. 36-5A). The use of indirect lighting can unmask subtle dimpling of the skin or nipple caused by a carcinoma that places Cooper’s ligaments under tension (see Fig. 36-5B). Simple maneuvers such as stretching the arms high above the head or tensing the pectoralis muscles may accentuate asymmetries and dimpling. If carefully sought, dimpling of the skin or nipple retraction is a sensitive and specific sign of underlying cancer.
Edema of the skin produces a clinical sign known as peau d’orange (see Fig. 36-5D). When combined with tenderness, warmth and swelling of the breast, these signs and symptoms are the hallmark of inflammatory carcinoma and may be mistaken for acute mastitis. The inflammatory changes and edema are caused by obstruction of dermal lymphatic channels with emboli of carcinoma cells. Occasionally, a bulky tumor may produce obstruction of lymph channels that results in overlying skin edema. This is not typically the case with inflammatory carcinoma, where there is usually no discrete palpable mass but diffuse changes throughout the breast parenchyma. In 40 patients with inflammatory carcinoma described by Haagensen,1 erythema and edema of the skin were present in all cases, a palpable mass or localized induration was noted in 19 patients, and no localized tumor was present in 21 patients.
A condition of the nipple that is commonly associated with an underlying breast cancer is Paget’s disease. First described by Sir James Paget in 1874, Paget’s disease has histologically distinct changes within the dermis of the nipple. There is often an underlying intraductal carcinoma in the large sinuses just under the nipple (see Fig. 36-5A). Carcinoma cells invade across the junction of epidermal and ductal epithelial cells and enter the epidermal layer of the skin of the nipple. Clinically, this produces a dermatitis that may appear eczematoid and moist or dry and psoriatic. It begins in the nipple, although it can spread to the skin of the areola. Many benign skin conditions such as eczema frequently begin on the areola, whereas Paget’s disease originates on the nipple and secondarily involves the areola.
Biopsy
Core Needle Biopsy
Interpretation of Core Needle Biopsy Results
The diagnosis of breast lesions using a minimally invasive procedure, such as core needle biopsy, is the preferred approach. The use of excisional breast biopsy as a diagnostic procedure increases costs and results in delays to definitive surgery for patients with cancer.2 Fewer than 10% of patients who undergo core biopsy will have inconclusive results and require wire-localized surgical biopsy for definitive diagnosis. Biopsy results that are not concordant with the targeted lesion (e.g., a spiculated mass on imaging and normal breast tissue on core biopsy) also require surgical excision. When ADH is found on core biopsy, surgical excision will reveal DCIS or invasive carcinoma in up to 20% of cases because of difficulty distinguishing ADH and DCIS in a limited tissue sample. A finding of a cellular fibroadenoma on core biopsy requires excision to rule out a phyllodes tumor.
Breast Imaging
Screening Mammography
Eight prospective randomized trials of screening mammography have been performed, with almost 500,000 women participating. The impact of mammographic screening on breast cancer mortality has been assessed by age group at specific intervals by the U.S. Preventive Services Task Force and the most recent report resulted in a change in recommendations for breast cancer screening. Based on the review of eight trials in women aged 39 to 49 years, screening mammography reduced the risk for breast cancer death by 15% (relative risk [RR], 0.85; credible interval [CrI], 0.75 to 0.96). In the six trials that included women aged 50 to 59, there was a reduction in risk of 14% (RR, 0.86; CrI, 0.75 to 0.99). There were two trials that included women aged 60 to 69 and, in this age group, there was a reduction in risk of 32% (RR, 0.68; CrI, 0.54 to 0.87). There was only one trial that included women older than 70 years, and it was concluded that there were insufficient data to recommend routine screening in this age group. Based on these results, the most recent U.S. Preventive Services Task Force report recommended biennial screening mammography for women aged 50 to 74 years and recommended against screening for those aged 40 to 49 and women older than 75 years of age.2 The recommendations were based on the risk reduction, number of women needed to invite for screening to prevent one breast cancer death, and potential for harm from additional testing and biopsies (Table 36-1).
Table 36-1 Effect on Breast Cancer Mortality and False-Positive Mammograms by Age Group in Breast Cancer Screening Trials
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree