The Normal Aorta
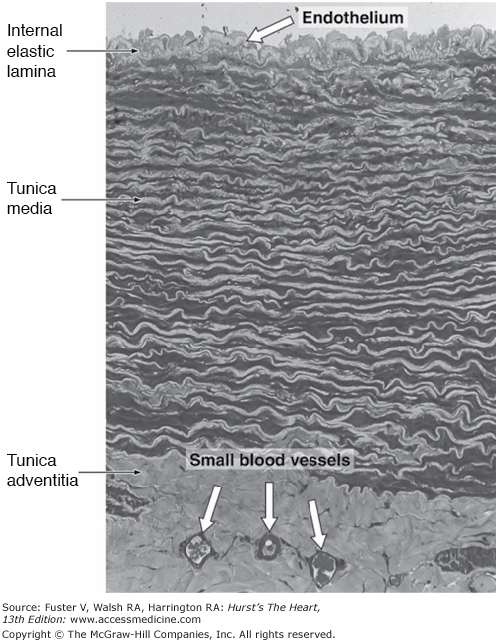
The aorta has a complex intrinsic biology and sophisticated mechanical properties involving intrinsic relaxation and contraction that interact with left ventricular ejection to enhance hemodynamic function. The major conductance vessel of the body, the aorta is an elastic artery with a trilaminar wall: the tunica intima, tunica media, and tunica adventitia (Fig. 106–1).1,2 The innermost lining of the tunica intima is the endothelium, resting on a thin basal lamina. The subendothelial tissue comprises fibroblasts, collagen fibers, elastic fibers, and mucoid ground substance. An internal elastic membrane forms the outer lining of the tunica intima. The tunica media is approximately 1 mm thick, comprising elastin, smooth muscle cells, collagen, and ground substance. The predominance of elastic fibers in the aortic wall and their arrangement as circumferential lamellae distinguish this elastic artery from the smaller muscular arteries. A lamellar unit is made up of two concentric elastic lamellae and the smooth muscle cells, collagen, and ground substance contained within.3,4 The thoracic aorta incorporates 35 to 56 lamellar units and the abdominal aorta about 28 units.5 Surrounding the tunica media is the tunica adventitia, which is composed of loose connective tissue, including fibroblasts, relatively small amounts of collagen fibers, elastin, and ground substance. The adventitia is known to surgeons as the “strength” layer of the aorta and is essential for secure suturing of aortic tissues. Within the tunica adventitia lie the nervi vasorum and vasa vasorum. The arteries arising along the course of the aorta give rise to the vasa vasorum, which develop into a capillary network supplying the adventitia and media of the thoracic aorta. The vasa vasorum do not supply the media of the abdominal aorta. Unlike the elastic fibers of the arterial wall, which are highly distensible, collagen is inelastic and provides the tensile strength required to prevent deformation and rupture of the aortic wall.
Figure 106–1.
Transverse section of the wall of a large elastic artery demonstrating the well-developed tunica media containing elastic lamellae. Pararosaniline–toluidine blue stain; medium magnification. Reproduced with permission from The circulatory system.1.
The ascending aorta is approximately 3 cm in diameter, depending on age, gender, and body surface area. The diameter of the aortic arch is similar. Descending in the posterior mediastinum, the thoracic aorta tapers slightly to about 2 to 2.3 cm. The abdominal aorta narrows to 1.7 to 1.9 cm in its distal portion. The aortas of males are larger than those of females, and aortic root dimension increases with age, height, and weight. The gender difference in aortic root dimension is not entirely explained by body surface area.5
The force of left ventricular (LV) ejection creates a pressure wave that traverses the aorta, producing radial expansion and contraction of the arterial walls.6 Potential energy derived from myocardial contraction and stored in the aortic wall during systole is transformed during diastolic recoil into kinetic energy, which drives blood into the peripheral vessels.7 The pressure wave is conducted at an approximate velocity of 5 ms, increasing in amplitude as it traverses the aorta.
Forward blood flow in the aorta begins when the aortic valve opens, and systolic pressure increases as the pressure wave courses along the length of the aorta. Flow velocity increases rapidly to a peak and then gradually decreases. With aortic valve closure, there is a transient period of backward flow before forward flow resumes during diastole, particularly in the descending thoracic and abdominal aorta, albeit at considerably less than systolic velocity. The incisura, present in the arterial waveform in the proximal portion of the thoracic aorta, gradually disappears and is generally absent in the abdominal aorta.8
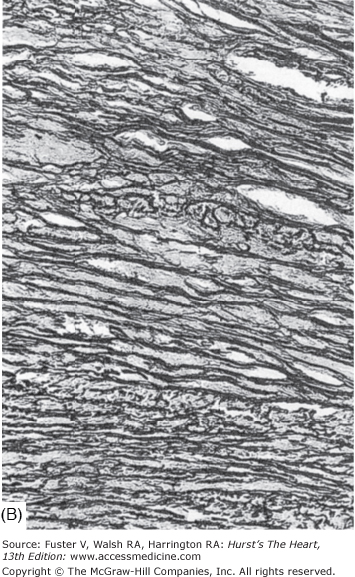
Each of the four components of the aortic wall—elastic tissue, collagen fibers, smooth muscle cells, and mucoid ground substance—change with age (Fig. 106–2).8 Elastic fibers fragment, collagen becomes more prominent at the expense of smooth muscle cells, and glycosaminoglycans accumulate. As a result, the aorta becomes less distensible, reducing its capacity to absorb the forces derived form LV contraction.9 Weakening of the aortic wall leads to dilatation of the lumen, as well as elongation and uncoiling of the aortic arch, collectively producing ectasia.
Figure 106–2.
Histology of the normal aorta of a child (A) and an elderly adult (B). With aging, elastic fibers fragment, collagen becomes more prominent, smooth muscle cells diminish, and acid mucopolysaccharide ground substance accumulates. Weakening of the aortic wall leads to dilatation of the lumen as well as elongation and uncoiling of the aortic arch. Orcein and van Gieson stain, magnification × 414. From Nichols and O’Rourke,8 with permission.
The aorta in elderly individuals is characterized by dilatation and elongation. Accompanying these changes are alterations in aortic wall structure, fragmentation of elastic fibers, loss of smooth muscle cell nuclei (medionecrosis), accumulation of collagenous tissue, and deposition of basophilic ground substance. As the aorta dilates because of disease of the medial layer, tension on the aortic wall increases shear stress. This process is accelerated by hypertension, particularly when pulsatile forces are high (see Precipitation of Aortic Dissection). Under conditions of increased peripheral vascular resistance, a reflected wave has an impact additive to that of antegrade pulsatile flow. The combination of inherited, degenerative, mechanical, and hemodynamic factors adversely affects the medial layer of the aortic wall, leading to dilatation and setting the stage for the catastrophes of aortic dissection or rupture.
Definitions and Categories
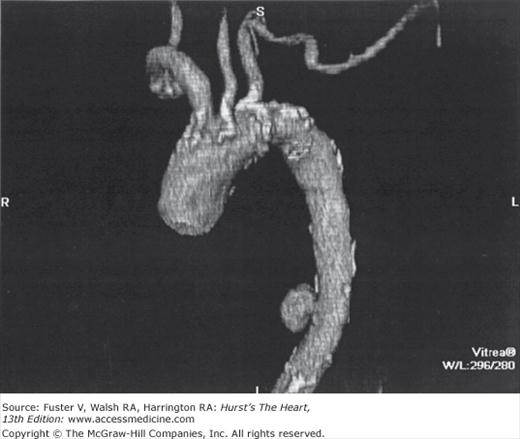
The aorta can be affected by a variety of pathologic processes leading to aneurysm, dissection, or ischemic syndromes (Fig. 106–3A). The term aneurysm—derived from the Greek aneurysma, referring to dilatation—is distinguished from ectasia, which refers to the modest generalized dilatation and elongation of the aorta that occurs with aging.10 The criterion for definition of small aneurysms is controversial. Although the size of the normal aorta varies with gender and body size, most agree that the maximum diameter of the thoracic aorta should not exceed 4 cm. For the abdominal aorta, which normally has a smaller diameter than the thoracic segments, it has been suggested that the term aneurysm be restricted to situations in which the diameter exceeds 3 cm.11 Another proposed definition depends on the affected segment having a diameter more than 1.5 to 2 times normal.12
Aneurysms may be classified according to their morphology, location, or etiology. In true aneurysms, the wall of the aneurysm is composed of the normal histologic components of the aorta. A false aneurysm, on the other hand, represents a contained rupture, and the wall does not contain the normal histologic components of the aorta. It is a fibrous peel that has formed from a small perforation of the aorta, initially controlled by adherence of surrounding tissues and gradually enlarging over time.
The gross morphologic classification distinguishes true aneurysms as fusiform (most common) or saccular. A fusiform aneurysm is roughly cylindrical and affects the entire circumference of the aorta; a saccular aneurysm is an outpouching of only a portion of the aortic circumference. Frequently, a small neck provides continuity between the aortic lumen and the saccular aneurysm.
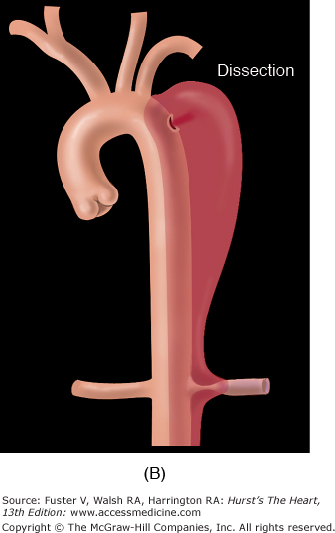
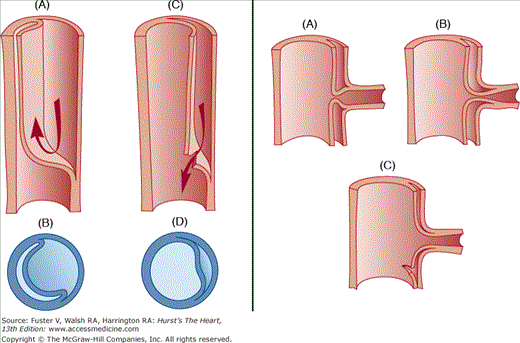
Aortic dissection refers to splitting of the layers of the aortic wall (within the media), permitting longitudinal propagation of a blood-filled space within the aortic wall (Fig. 106–3B). Aortic dissection is considered the most common cause of death as a result of human aortic disease.13Atherosclerosis of the aorta can narrow the ostia of the great vessels or produce mobile intraaortic atheromatous masses capable of embolization to the brain or other organs.
Several additional entities should be distinguished (Fig. 106–4). Acute aortic transection is a consequence of trauma and involves localized disruption of the aortic wall, which is intrinsically normal and resistant to propagating dissection. A ruptured aortic aneurysm is self-explanatory unless an acute aortic transaction or acute aortic dissection happens to rupture, which are common developments. Acute aortic dissection refers to the separation of layers of the aortic wall discussed fully below. Intramural aortic hematoma is akin to circumferential rather than longitudinal dissection, such that there is no identifiable flap separating true and false lumens. Penetrating aortic ulcer involves localized perforation of the medial layer of the aortic wall at the site of an atherosclerotic plaque.
Aortic aneurysms may also be classified according to the segment involved—as thoracic, thoracoabdominal, or abdominal. Whereas aneurysm formation can be considerably more widespread along the length of the aorta (Fig. 106–5) than obstructive atherosclerotic disease, potentially affecting almost the entire vessel, obstruction tends to involve only the abdominal portion of the aorta, when the iliac arteries are commonly affected as well. Dilatation of the aorta may occur as a consequence of atherosclerosis alone, as well as of aging, infection, inflammation, trauma, congenital anomalies, or medial degeneration or in combinations of pathologic states. The pathologic changes that accompany these conditions cause the aorta to thicken, thin, bulge, tear, rupture, narrow, or dissect or to be altered by combinations of these conditions.
Over the course of normal aging, degenerative changes occur throughout most of the length of the aorta, leading to a mild form of what is termed cystic medial necrosis. Although essentially a normal physiologic process of aging, cystic medial necrosis develops more rapidly in patients with bicuspid aortic valve, during pregnancy, and very markedly in people with Marfan syndrome. The mechanism by which the medial layer of the aorta is subject to this accelerated rate of degeneration is a subject of active molecular genetic investigation. Severe elastic fiber degeneration, necrosis of muscle cells, and cystic spaces filled with mucoid material are most often encountered in the ascending aorta from the region of the valve to the brachiocephalic artery (see Fig. 106–5). Because of the dilatation of the aortic root, aortic regurgitation may be a secondary feature, although the valve leaflets themselves are histologically unaffected.
Cystic medial necrosis is the most common cause of ascending aortic aneurysm,13 and although this type of aortic pathology is typical of patients with the Marfan syndrome, it may also occur in the absence of any clinical Marfan stigmata.
Aneurysms
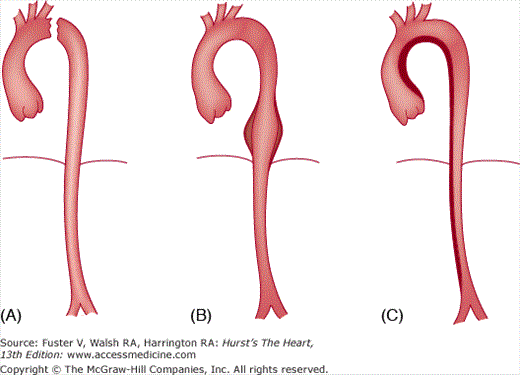
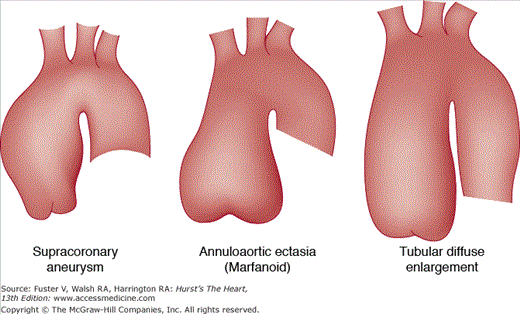
Ascending aortic aneurysms occur more frequently in men than in women, are typically fusiform, and extend into the aortic arch. Consequently, aneurysms of the aortic arch are often contiguous with aneurysms in the ascending aorta. Ascending aortic aneurysms are divisible into three categories according to the pattern of involvement of the aortic root (Fig. 106–6), with direct implications for surgical treatment. The most common type is supracoronary aneurysm, in which the aortic annulus is normal in size, as is the short segment of aorta between the annulus and the coronary ostia. This type is well-treated by a tube graft, starting above the coronary arteries and extending cephalad to the end of the aneurysm. The second category is termed annuloaortic ectasia, calling attention to the enlargement of both the aortic annulus and the proximal portion of the aorta. This type of aneurysm is typical of Marfan syndrome and other related disorders characterized by cystic medial necrosis of the aortic wall.15 Because the annulus and proximal aorta are the most dilated portions, the aneurysmal ascending aorta takes on a “flasklike” shape, requiring replacement of the entire aortic root and valve, usually with a prefabricated composite graft that includes a prosthetic valve and aortic graft in an integrated unit. The third category, the tubular type of ascending aortic aneurysm, has features midway between the other two configurations. In patients with tubular aortic aneurysms, the annulus and proximal aorta are mildly dilated, and the caliber of the ascending aorta is more uniform. When surgical repair is necessary, either a supracoronary tube graft or total aortic root replacement may be appropriate based on such considerations as patient age. In younger individuals, composite grafting may confer greater protection against late dilatation of the proximal portion of the aortic root.
In contrast to the ascending aorta, the majority of aneurysms of the descending thoracic aorta are atherosclerotic.16 These are typically fusiform, may extend to the level of the abdominal aorta, and often begin distal to the origin of the left subclavian artery. Descending thoracic aneurysms may also develop in patients with aortic coarctation.
In more than 90% of cases, the superior margins of abdominal aneurysms are distal to the renal arteries. Atherosclerosis has been held responsible for the majority, although some authors suggest that atherosclerosis may be a secondary phenomenon in aneurysmal disease.
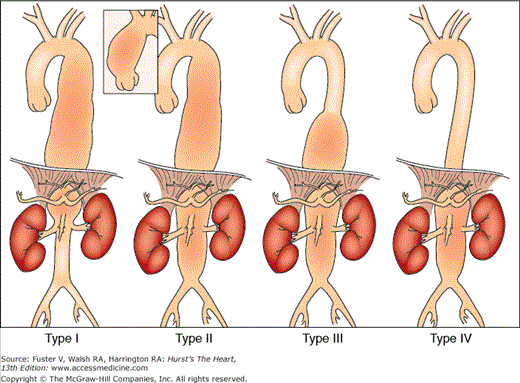
As suggested by the nomenclature, thoracoabdominal aortic aneurysms have features of both thoracic and abdominal aortic aneurysms (Fig. 106–7). Although they constitute only approximately 3% of all aortic aneurysms, thoracoabdominal aneurysms are considered as a separate class because of the diffuse and extensive aortic involvement in the disease process (usually atherosclerosis) and special considerations for surgical repair, which may entail reimplantation of the origins of visceral arteries.17 Crawford and Coselli18 delineated four types of thoracoabdominal aortic aneurysms, according to the segment and extent of aorta involved, and which are described in Fig. 106–7.18
Figure 106–7.
Classification of thoracoabdominal aortic aneurysms. Type I aneurysms involve most of the descending aorta from near the origin of the left subclavian artery to the abdominal vessels, but the renal arteries are not involved. Type II aneurysms also begin near the origin of the left subclavian artery but extend below the origins of the renal arteries. Type III aneurysms arise more distally and involve less of the descending thoracic aorta but often more of the abdominal aorta than types I and II aneurysms. Type IV aneurysms arise at the level of the diaphragm and typically extend below the origins of the renal arteries. From Crawford and Coselli,18 with permission.
Epidemiology of Aneurysmal Disease
The epidemiology of aortic aneurysms and their corresponding acute aortic syndromes presents methodologic challenges because of confusion in terminology, referral bias, unknown incidence of asymptomatic cases, misdiagnosis of aortic rupture or dissection as myocardial infarction (MI), and limitations of administrative databases. Despite these considerable limitations, multiple studies have assessed the epidemiology of abdominal and thoracic aortic aneurysms.19-21 The most recent data available from the Centers for Disease Control and Prevention (CDC) list aneurysmal disease as the 17th most common cause of death in all individuals and the 15th most common in individuals older than 65 years of age.20
Abdominal aortic aneurysm affects approximately 5% of individuals older than age 65 years, and the prevalence is considerably higher in men than women.21 The annual incidence of ruptured abdominal aortic aneurysm is approximately 10 per 100,000 population and is similar in men and women. However, the age at diagnosis is a decade higher in women.22,23 For men in their 60s, the rate increases to approximately 50 per 100,000, and for those in their 70s, the rate exceeds one in 1000 men experiencing rupture of abdominal aortic aneurysms each year.
The incidence of thoracic aortic aneurysm is also approximately 10 per 100,000 population and is similar in women and men, but the age at diagnosis is a decade higher in women (70s). The yearly incidence of rupture or dissection of an existing thoracic aortic aneurysm is approximately 7%, divided equally between rupture and dissection. A curious and as yet unexplained finding is that 79% of thoracic aortic aneurysm ruptures occur in women.22,23
Aortic aneurysm and its acute complications are recognized with increasing frequency. These trends during the past 3 decades have been confirmed by the CDC for the United States, Scotland, the Netherlands, England, and Wales.24
The epidemiologic implications of screening for abdominal aortic aneurysms have been extensively studied.21 Screening programs based on abdominal ultrasonography clearly detect aneurysms effectively and reduce the number of aneurysm-related deaths. The cost of medical care per single aneurysm-related death prevented is about $10,000. One important benefit of screening is that a single negative ultrasonogram at 65 years of age suffices; patients without aneurysms at this age simply will not die of aneurysm rupture.21
Cost-effective screening of the general population is focused on family history of aneurysmal disease, age, male gender, history of cigarette smoking, coronary artery disease, cerebrovascular disease, and hypercholesterolemia as factors linked with incremental risk for aneurysm development. Abdominal aortic aneurysm is five times more common in smokers, in whom 89% of all aneurysm ruptures occur.21
The U.S. Preventive Services Task Force recommends a onetime screening for abdominal aortic aneurysm by ultrasonography in men ages 65 to 75 years who have ever smoked. There is no recommendation (for or against) screening in men age 65 to 75 years who have never smoked and an explicit recommendation against routine screening for abdominal aortic aneurysm in women based on the relatively low yield.
Patients with abdominal aneurysms may also harbor thoracic aortic aneurysms and vice versa. Very recently, preliminary evidence has been presented that patients with thoracic aneurysms have approximately a 10% likelihood of concurrent intracranial aneurysm (incidence higher for descending than for ascending aneurysm patients).25 These intracranial aneurysms are of great importance to patients’ quality and duration of life. Based on this emerging evidence, routine screening for intracranial aneurysm is probably warranted. The clinical finding of concurrence of intracranial with thoracic aortic aneurysm is supported by common genetics and pathophysiologic mechanisms.
Etiology and Pathophysiology
This inherited disorder, described in 1896, is characterized primarily by dolichostenomelia (long, thin extremities), ligamentous redundancy, ectopia lentis, ascending aortic dilatation, and incompetence of the aortic or mitral valves (or both).26 A large number of specific mutations (>600) produce the Marfan phenotype, thus reducing the clinical usefulness of genetic testing in the diagnosis of Marfan syndrome.27 Diagnosis is made largely on a clinical basis using the criteria summarized in Table 106–1. The syndrome is linked to an autosomal dominant anomaly in the genes regulating synthesis of fibrillin type 1, a large glycoprotein that helps direct and orient elastin in the developing aorta.28 Mutations have been identified both on chromosome 15 and on chromosome 5. It is not clear whether these mutations lead to a qualitative or quantitative defect of elastin, or both, but there is a link between the pathology of the aortic media and the identification of specific genetic mutations involving these two chromosomes.27,28
| Cardiovascular | Skeletal System | Ocular System | Pulmonary System | Skin and Integument | Dura | Family History/Genetics | |
|---|---|---|---|---|---|---|---|
| Major criteria | One required: | Four required: | Ectopia entis | None | None | Dural ectasia | Parent, child, or sibling with Marfan disease |
Dilatation of Asc A(+ Al), involving sinuses of Valsalva AAD | Pectus carinatum Pectus excavatum (requiring surgery) Reduced upper-to-lower segment ratio or increased arm span-to-height ratio Positive wrist and thumb signs Elbow extension reduced <170° Pes planus Protrusion acetabulae | FBN1 mutation | |||||
| Minor criteria | MVP (± prolapse) Dilatation of the main PA (patients younger than 40 y) | Pectus excavatum (moderate) Hypermobile joints Crowding of teeth or highly arched palate | Flat corneas Increased axial length of globe Hypoplastic iris or ciliary muscles, causing decreased meiosis | Spontaneous pneumothorax Apical blebs | Striae Recurrent or incisional hernias | None | None |
| Involvement (required to say organ system involved) | One major or one minor criterion | Two major or one major and one minor criteria | One major or two minor criteria | One minor criterion | One minor criterion | Major criterion | Major criterion |
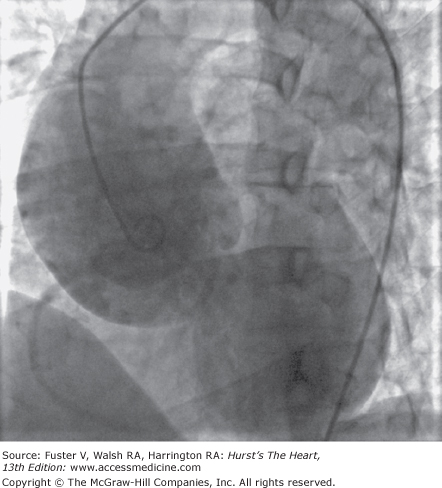
In patients with the Marfan syndrome, the aortic root tends to enlarge in fusiform fashion in association with aortic valvular regurgitation, and about half of patients also have mitral insufficiency (Fig. 106–8). Aneurysms involve the sinuses of Valsalva and the tubular portion of the ascending aorta, producing annuloaortic ectasia characterized by degeneration of elastic fibers and accumulation of mucoid material within the medial layer of the aortic wall (Fig. 106–9). Abnormalities associated with Marfan syndrome typically affect the entire length of the aorta, although dissection most often involves the thoracic portion.29
Figure 106–8.
Aortogram of a patient with Marfan syndrome. Aortic aneurysms in this disorder are characterized by annuloaortic ectasia involving the sinuses of Valsalva and the ascending aorta produced by degeneration of elastic fibers and the accumulation of mucoid material within the medial layer of the aortic wall, grossly resembling cystic medial necrosis.
Another inherited connective tissue disorder associated with aneurysm formation in Ehlers-Danlos syndrome. This disease is seen in clinical practice much less frequently than Marfan syndrome.
A recently recognized syndrome is the development of aneurysms caused by mutations in the transforming growth factor β (TGF-β) receptor (Loeys-Dietz syndrome). This has a phenotypic overlap with the vascular form of Ehlers-Danlos syndrome, and individuals with this mutation develop aneurysms that enlarge rapidly and are prone to rupture; in one series, the mean age at death was 26 years.30
Patients born with bicuspid aortic valve have structural abnormalities of the ascending aorta predisposing them to aneurysm and dissection.31-34 Some have aortic coarctation as part of this syndrome as well. It is important to recognize that aneurysmal enlargement of the aorta in patients with bicuspid aortic valve may occur before the onset of aortic stenosis or regurgitation and is not, therefore, predominantly a result of poststenotic dilatation. Because bicuspid valve disease is so common (affecting ∼2% of the population), the associated aortic disease is responsible for more cases of dissection than the more commonly appreciated Marfan syndrome (which affects one in 10,000 people) (Table 106–2).
| Incidence | AAD Likelihood (Lifetime) (%) | AAD Caused (as % of Population) (%) | |
|---|---|---|---|
| Marfan disease | 1/10,000 (0.01%) | 40 | 0.004 |
| Bicuspid aortic valve | 2/100 (2%) | 5 | 0.1 |
It is estimated that bicuspid valve disease accounts for more morbidity and mortality than all other congenital cardiac lesions combined.35 Many authorities believe that this disease is so virulent that every individual with a bicuspid aortic valve will, given enough time, develop aortic stenosis, aortic insufficiency, or aortic aneurysm or dissection related to the bicuspid valve disease. Ascending aortic aneurysm in patients with bicuspid aortic valve is currently considered nearly as serious as ascending aortic aneurysm in patients with Marfan disease, and operation is often offered earlier than in nonbicuspid patients. Evidence now points to a congenital pattern of inheritance, and the fundamental embryology is being elucidated in animal models. Evidence shows that excess matrix metalloproteinase (MMP) activity characterizes both the valve tissue and the aortic wall in bicuspid patients. The aorta grows more rapidly in bicuspid patients. Especially when there is associated valvular dysfunction, the current trend favors aggressive aortic resection in bicuspid patients with ascending aortic aneurysms.
Many cases that were once considered idiopathic or attributed to atherosclerosis can now be traced to genetic or metabolic abnormalities affecting the aortic wall. In many cases of nonmarfanoid aortic aneurysm, there is a family history of aneurysmal disease. When the proband has a thoracic aortic aneurysm, the chance that a relative has an aortic aneurysm is one in five.36 An autosomal dominant pattern of inheritance predominates, with reduced penetrance, but other genetic patterns also are manifest (Fig. 106–10). Probands with ascending aortic aneurysm are more likely to have family members with ascending aneurysm, and those with aneurysms involving the descending aorta are more likely to have family members with abdominal aneurysms.
Multiple genetic mutations have been linked with familial aortic aneurysms,37,38 including the thoracic aortic aneurysm and dissection 1 (TAAD1) mutation, which accounts for 20% to 30% of familial cases; the TGF-β receptor 2 (TGF-βR2) mutation, which accounts for approximately 5% of cases; the familial aortic aneurysm 1 (FAA1) mutation; and the myosin heavy chain 11 (MYH11) mutation. Much attention is currently focused on the TGF-β pathway and its potential inhibition by losartan, the angiotensin II receptor blocker.39 A multicenter, randomized trial in pediatric patients with Marfan disease is underway.
Although in many patients, genetic mutations establish the propensity for aneurysm development, gene substitution may set the stage, and lytic MMP enzymes degrade the structural proteins of the aortic wall, leading to aneurysm formation and dissection (Fig. 106–11). These enzymes are normally regulated by tissue inhibitor of metalloproteinase (TIMP), which antagonizes the lytic action of the MMP. An uncertain proportion of patients with aortic aneurysms manifest excessive MMP activity, but such activity has been documented in cases of abdominal aortic aneurysm,40,41 as well as in cases of ascending aortic aneurysm and aortic dissection.42 Several dozen specific MMP enzymes and certain TIMPs have been implicated in the pathogenesis of aneurysm disease.43
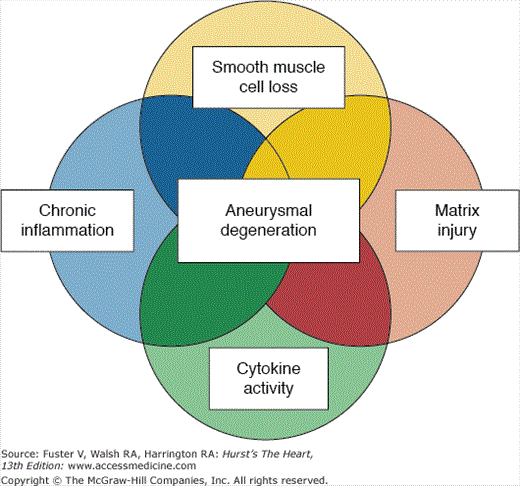
In addition to proteolysis of the extracellular matrix, the pathogenesis of aortic aneurysms involves inflammation, cytokine activity, and loss of smooth muscle cells. Together, these processes undermine the integrity of the aortic wall and set the stage for aneurysm formation, growth, and rupture. Inflammatory cells participate in the genesis of aneurysms.43-45 Smooth muscle cells of the aortic wall are charged with repairing damaged proteins, and substantial (∼75%) reduction in the number of smooth muscle cells in the walls of abdominal aortic aneurysms impairs this function. Aneurysmal smooth muscle cells display a three-fold increase in apoptosis and impaired growth capacity. Fig. 106–12 illustrates the interaction of proteolysis, inflammation, cytokine activation, and deficient smooth muscle cell function in the pathophysiology of aneurysm formation.
Many patients with ascending aortic aneurysms, which are typically nonatherosclerotic, display relatively little evidence of systemic atherosclerotic disease. A study comparing patients with ascending aortic aneurysm associated with annuloaortic ectasia or aortic dissection with age- and gender-matched control subjects found significantly less arterial calcification as detected by computed tomography (CT) imaging in those with aneurysms. This implies that the mutation responsible for ascending aortic aneurysm may have protective value against atherosclerosis, and certain MMPs that exert dilatory effects on the aortic wall may have antiatherogenic properties as well.
Clinical Manifestations
Unfortunately, the vast majority of thoracic and abdominal aortic aneurysms are clinically silent, with rupture constituting the first manifestation.46 A minority of patients (5%-10%) experience symptoms, permitting earlier detection of the aneurysm. Aneurysms of any kind can produce pain arising from stretching of the aortic tissue or impingement on adjacent structures. Pain originating in the ascending aorta is usually felt retrosternally. Pain from the descending aorta is characteristically located between the scapulae. Pain in the lateral or posterior chest may occur when the aneurysm compresses surrounding structures or erodes into adjacent ribs or vertebrae. Pain from the abdominal aorta may occur in the abdomen, left flank, or lower part of the back. It is often difficult to distinguish aneurysm pain from that resulting from other causes, but patients may distinguish deep visceral pain from superficial or musculoskeletal pain. The interscapular location of pain caused by descending aortic enlargement or dissection is less often caused by musculoskeletal disease.
Rupture of the aorta in any location produces acute symptoms, usually severe pain followed by loss of consciousness or death as a result of internal hemorrhage. Rupture of an abdominal aortic aneurysm usually produces the clinical picture of extreme distress as a result of an abdominal catastrophe. Despite surgical advances, mortality is still the most frequent outcome because abrupt circulatory collapse prevents timely intervention except in unusual circumstances. Patients frequently have severe abdominal or back pain, but the pattern varies considerably. The aneurysm may rupture into the retroperitoneum or into the peritoneal or pleural cavities, leading to tachycardia, hypotension, diaphoresis, pallor, or shock, depending on the extent of rupture and associated blood loss into the extravascular space. On occasion, rupture occurs directly into the duodenum, causing an aortoduodenal fistula and acute gastrointestinal bleeding. This possibility should be considered when gastrointestinal bleeding is evident along with signs of an aneurysm on physical examination. Rupture may also occur into the inferior vena cava or iliac veins, producing an arteriovenous fistula; this is suggested by rapid development of leg swelling or high-output heart failure in the presence of an abdominal aortic aneurysm. Rupture of a descending or thoracoabdominal aortic aneurysm produces similar physiologic derangements, with the associated pain typically located higher in the trunk, consistent with the anatomical location of the disease process.
Aside from pain, ascending aortic aneurysms may produce heart failure on the basis of incompetence of the aortic valve. As the aortic root enlarges, diastolic coaptation of the aortic valve leaflets progressively falters, causing regurgitation of blood through the resultant central gap. Aneurysms of the sinuses of Valsalva may rupture directly into the right ventricular cavity, right atrium, or pulmonary artery, causing heart failure associated with a continuous murmur. Ascending aortic aneurysms can distort or obstruct the trachea, producing respiratory symptoms. Compression of the superior vena cava may produce venous congestion in the head, neck, and upper extremities. Aortic arch aneurysms or descending thoracic aortic aneurysms may produce hoarseness or dysphagia (“dysphagia lusoria”) from distortion of the recurrent laryngeal nerve or direct impingement on the esophagus. Descending thoracic aortic aneurysms may cause hemoptysis from direct erosion into the lung parenchyma or bronchi or hematemesis from esophageal erosion. Because mural thrombosis is so common in atherosclerotic aneurysms, they may be the source of peripheral embolism of thrombus or atherosclerotic debris causing occlusion of distal vessels or clinical features of atheroembolism. Occasionally, patients with abdominal aortic aneurysms may become aware of prominent abdominal pulsation. Nausea and vomiting may occur if an aneurysm compresses the duodenum. Compression of the left iliac vein may cause left leg swelling, compression of the left ureter may cause hydronephrosis, compression of the testicular veins may cause varicocele, or compression of the bladder may cause urinary frequency or urgency.
Thoracic or abdominal aortic aneurysms are most commonly diagnosed not on the basis of symptoms but incidentally by imaging procedures carried out for another reason. Occasionally, chest radiographs obtained to evaluate pulmonary symptoms or for general screening suggests an aortic aneurysm, which may then be confirmed by CT or magnetic resonance imaging (MRI).
Aortic dissection typically produces sudden intense pain, often described as tearing or shearing in quality. Whereas ascending aortic dissection usually causes anterior, substernal chest pain, dissection of the descending aorta causes posterior, interscapular pain. Pain may migrate inferiorly to the flank or pelvis as the dissection propagates distally. Impending aortic rupture should be considered when pain subsides and later recurs.45
Painless dissection occurs in as many as 15% of patients46 and is commonly detected as an asymptomatic finding on elective CT scans obtained for another purpose. By convention, aortic dissection is considered acute when identified within 2 weeks of onset and chronic when symptoms or other markers of dissection have been present longer.
Clinically, the inciting event responsible for aortic dissection is an intimal tear that permits blood to pass from the true lumen into the middle or outer layer of the aortic media, forming a second or false lumen separated by an intimal flap.47 In some cases, intramural hematoma precedes perforation of the intima, possibly related to rupture of a vasa vasorum.48 The dissection may propagate distally (antegrade) or proximally (retrograde) to narrow or occlude the origin of any branch artery arising from the aorta. Antegrade propagation is more common, and the dissection usually has a spiral morphology, leaving some aortic branches supplied by the true lumen and others in continuity with the false lumen (see Fig. 106–11).
The intimal tear originates in the ascending aorta in 65% of cases, transverse arch in 10%, upper descending aorta just beyond the origin of the left subclavian artery in 20%, and more distally in 5% of cases. The false lumen may terminate at any point along the length of the aorta or in the iliac or femoral arteries, and there are sometimes multiple flaps and several sites of reentry. The false lumen can undergo retrograde dissection, thrombotic occlusion, pseudoaneurysm formation, compression, or rupture.
Aortic dissection occurs more often in men than in women, with a 2–5:1 preponderance,46 usually in the sixth or seventh decades of life. Systemic hypertension is the major predisposing risk factor. In the International Registry of Acute Aortic Dissection, 194 of 289 patients (69%) with proximal dissections and 132 of 175 (77%) with distal dissections had a history of hypertension; similar ratios were reported by investigators at the Mayo Clinic.46 Other predisposing conditions include Marfan syndrome, Ehlers-Danlos syndrome, Loeys-Dietz syndrome, bicuspid aortic valve, aortic aneurysm, and annuloaortic ectasia associated with cystic medial necrosis. The most common causes of aortic dissection in patients younger than 40 years of age are Marfan syndrome and pregnancy. Iatrogenic trauma resulting from intravascular catheterization is the cause in approximately 5% of cases and may involve the ascending, descending, or abdominal aorta. Cocaine abuse is increasingly recognized as a factor predisposing to acute aortic dissection.49
Aortic dissection is often fatal unless early diagnosis is made and aggressive treatment implemented. Because the presenting symptoms and signs are myriad and nonspecific, dissection may be initially overlooked in up to 40% of cases. The diagnosis is made only at postmortem examination in a disturbingly large fraction of cases. Few other conditions demand such prompt diagnosis and treatment because the mortality rate of untreated aortic dissection approaches 1% to 2% per hour during the first 48 hours, reaching 90% at 3 months, and most deaths directly related to dissection occur within the first 14 days after onset.50,51 Up to 20% of victims die before reaching hospital.50 With expert care, however, survival of approximately 70% of patients reaching the hospital with acute aortic dissection has been reported. Most patients require intensive medical therapy, either as the sole treatment or as a stabilizing measure until surgical therapy is undertaken. Even if the patient survives an aortic dissection, the aneurysm then expands more rapidly than the nondissected aortic aneurysm, contributing to shortened survival.
Dissections of the thoracic aorta may occur in the ascending (type A) or descending (type B) segments, determined by the location of the inciting intimal tear. Tears generally occur in two specific locations: the ascending aorta, 2 to 3 cm above the coronary arteries, and in the descending aorta, 1 to 2 cm beyond the origin of the left subclavian artery. The first type produces ascending dissection and the second descending dissection, but dissections originating in the ascending aorta commonly extend along the aortic arch to involve the descending and abdominal portions of the aorta as well.
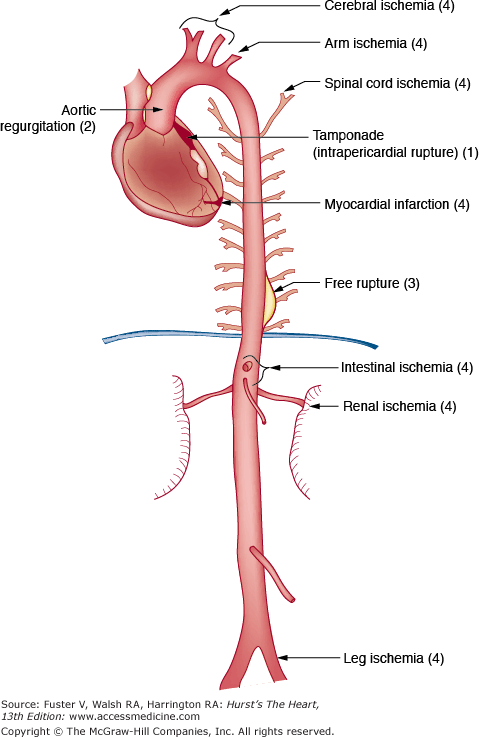
Aortic dissection can cause death in four main ways (Fig. 106–13):
- Hemopericardium with cardiac tamponade caused by retrograde dissection of an ascending aortic dissection
- Acute aortic insufficiency caused by retrograde dissection of an ascending aortic dissection
- Rupture of a descending aortic dissection into the pleural space
- Occlusion of a branch artery with consequent tissue ischemia
Fig. 106–14 illustrates the mechanism of branch occlusion and the benefits of spontaneous or surgical fenestration.
Figure 106–14.
Schematic representation of the distended false channel in a case of aortic dissection impinging on the lumen of a branch vessel. Left. Main aortic trunk, with A and B before fenestration and C and D after fenestration. Right. Anatomic events at branch vessel; A and B show impingement by a false lumen, and C shows relief by fenestration. From Crawford and Crawford,18 with permission.
Most patients with aortic dissection present with hypertension, but 3% to 18% present with shock, sometimes secondary to extension of dissection into the coronary arteries, acute MI, LV failure, acute severe aortic insufficiency, cardiac tamponade, or aortic rupture. Coronary perfusion may be compromised by retrograde dissection, compression by the false lumen, or hypotension. In one series, differential pulse volume and blood pressure between the right and left upper extremities was detected in 38% of patients with ascending aortic dissection. An abrupt loss of pulse may affect the carotid, subclavian, axillary, radial, ulnar, or femoral arteries, and acute limb ischemia has been reported in 20% of patients. Branch vessel occlusion results from compression by the distended false lumen of the true lumen of the branch vessel.
Approximately 15% to 20% of patients with aortic dissection develop neurologic deficits, with transient cerebral ischemia or stroke in up to 10% of patients resulting from extension of dissection into the carotid or vertebral arteries. In such cases, brain imaging and neurologic or neurosurgical consultation may be helpful to determine whether cerebral infarction has occurred; surgery is best avoided in such cases for fear of inducing intracerebral bleeding or otherwise extending the zone of infarction. When cerebral infarction is absent or incomplete, urgent operation is generally indicated because repair of the dissection may restore brain perfusion. Similarly, urgent surgical intervention is indicated when interruption of spinal circulation by a dissection of the descending aorta threatens to cause paraplegia.
Aortic rupture is the most common cause of death in patients with aortic dissection. The second most common cause of death in these patients is acute, severe aortic regurgitation, which has been reported in 44% of patients with dissection of the ascending aorta and is poorly tolerated hemodynamically, compared with chronic aortic insufficiency; because sudden volume overload allows no time for LV adaptation, cardiogenic shock typically ensues.
Intramural aortic hematoma differs from typical dissection in that there is no flap delineating the true and false lumens, and the hematoma is located circumferentially around the aortic lumen, rather than obliquely (Fig. 106–15).52 Whether the intramural hematoma arises from a small intimal tear that is not radiographically detected or from a rupture of a vasa vasorum within the aortic wall remains controversial. The clinical course is variable; the hematoma may persist, resorb (returning the aorta to a normal appearance), leave an aneurysm with the possibility of rupture, or later result in dissection.
Penetrating aortic ulcer involves disruption of the internal elastic lamina and erosion of the medial layer of the aortic wall, resulting in local penetration at the site of an atherosclerotic plaque. This lesion may mimic or result in aortic dissection, pseudoaneurysm formation, intramural hematoma, or rupture (Figs. 106–15, 106–16, 106–17).53
It is important to recognize that intramural hematoma and penetrating aortic ulcer are diseases associated with advanced age, characteristically occurring in patients older than those with type B aortic dissection. In addition, although branch vessel occlusion is commonly associated with aortic dissection, this does not occur as a consequence of penetrating aortic ulcer or intramural hematoma.
The management of patients with penetrating aortic ulcer or intramural hematoma is controversial. When the descending aorta is involved, most authorities advocate medical management with pharmacologic therapy aimed at plaque stabilization and reducing systolic arterial wall stress. Others take a more aggressive stance, operating on all patients except very old and infirm ones, based on observational follow-up of unoperated patients, many of whom die of aortic rupture within 1 to 2 years.
For intramural hematoma involving the ascending aorta, there is more unanimity favoring immediate surgical intervention, although the Japanese literature challenges the need for routine surgery, even in this anatomic location.54 Penetrating aortic ulcers usually involve the descending aorta distal to the origin of the left subclavian artery, and for those associated with persistent pain, stent grafting is the treatment of choice. Figure 106–16 illustrates a dramatic case in which a penetrating ulcer ruptured through the posterior wall of the ascending aorta, mimicking “cryptogenic” pericardial effusion until surgical exploration made the diagnosis clear.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree