and Alessandra Cancellieri2
(1)
Department of Radiology, Bellaria Hospital, Bologna, Italy
(2)
Department of Pathology, Maggiore Hospital, Bologna, Italy
Radiology | Nicola Sverzellati Mario Silva |
Amyloidosis, interstitial | Amyloidosis | Page 56 |
ECD | Erdheim-Chester disease | Page 58 |
LC | Lymphangitic carcinomatosis | Page 60 |
PE, interstitial | Pulmonary edema, interstitial | Page 62 |
VOD | Veno-occlusive disease | Page 64 |

Amyloidosis, Interstitial
Definition
Amyloidosis is a group of diseases caused by extracellular accumulation of abnormal misfolded autologous proteins (amyloid deposit), in a variety of organs and tissues. Current classification of amyloidosis is based on the type of fibrillar protein in the amyloid deposit (fibrillar amyloid light chain, AL, and serum amyloid A, AA, are the most common).
Amyloidosis can be primary or secondary in origin; hereditary forms are described but usually do not involve the chest. Localized and systemic forms of amyloidosis are described, with two main patterns of pulmonary involvement at HRCT, namely, nodular or diffuse. The interstitial diffuse form is more symptomatic than the nodular one.
Interstitial amyloidosis
Berk JL (2002) Pulmonary and tracheobronchial amyloidosis. Semin Respir Crit Care Med 23(2):155
High-Resolution CT: HRCT
Key Signs
Nodular interlobular septal thickening (beaded septum sign)
Smooth interlobular septal thickening ( )
)
Thickening of the bronchovascular bundles (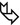 )
)
Nodules (calcified in up to 50 % of cases) (see the image with mediastinal window)
Distribution
Usually symmetric and diffuse, but selective involvement with segmental distribution can be seen. Reticular opacities are predominantly subpleural and basal.
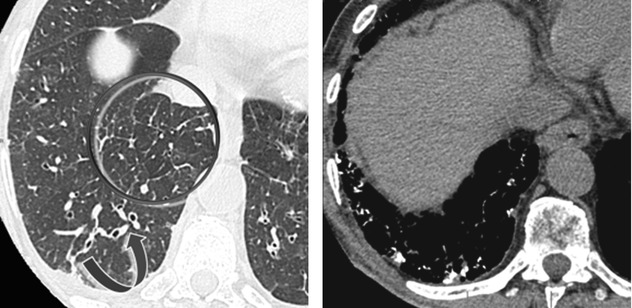
The beaded septum sign consists of nodular thickening of interlobular septa reminiscent of a row of beads. The beaded septum sign was initially described as a sign of lymphangitic spread of cancer although other diseases may be responsible of this sign (please also refer this sign in chapters “Septal Pattern” and “Case-Based Glossary with Tips and Tricks”).
The reticular and nodular findings might be unspecific. The identification of coexisting nodules may help radiologists suggest the correct diagnosis. Nevertheless, pulmonary edema should always be considered in differential diagnosis, notably because it is frequent in subjects with myocardial amyloid deposits.
Pickford HA (1997) Thoracic cross-sectional imaging of amyloidosis. AJR Am J Roentgenol 168(2):351
Ancillary Signs
Ground-glass opacity.
Patchy bilateral consolidations could show calcifications, some of them with punctate aspect.
Lung cysts may coexist (➨), particularly in cases associated with lymphoproliferative disorders (e.g., LIP).
Non-parenchymal Signs
Pleural thickening may be associated with pleural effusion (►).
Hilar and mediastinal lymphadenomegaly with calcification is common in AL form of amyloidosis but uncommon in AA variant.
Myocardial infiltration (wall thickening of the left ventricle with systolic and diastolic dysfunction and subendocardial or transmural late enhancement at MRI).
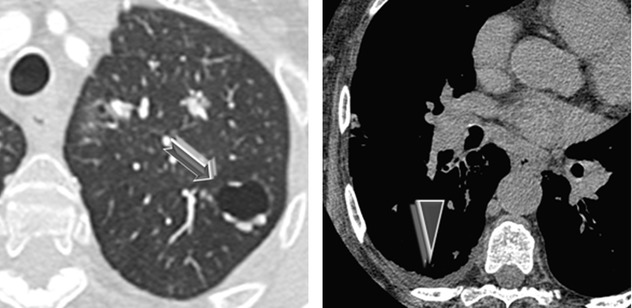
Differential diagnosis includes sarcoidosis because of beaded reticulation associated with lymph node enlargement with coarse calcifications. Also, it is important to differentiate between interstitial involvement from systemic amyloidosis and localized forms of diffuse alveolar septal amyloidosis, because the latter has more severe prognosis from pulmonary impairment.
Cordier JF (2009) Pulmonary amyloidosis in hematological disorders. Semin Respir Crit Care Med 26(5):502
Boydking A (2009) Localized interstitial pulmonary amyloid: a case report and review of the literature. Curr Opin Pulm Med 15(5):517–520
Course and Complications
Treatment of underlying disease warrants resolution of interstitial involvement in the majority of cases of AA amyloidosis.
AL amyloidosis related to hematologic disorders resolves in 30 % of cases treated with stem cell transplantation.
Clinical and radiological findings of the lung are usually secondary in pulmonary involvement from systemic amyloidosis. Cardiac involvement is a major prognostic factor; complications of cardiac involvement can overlap the pattern of interstitial amyloidosis. Assessment of myocardial involvement is suggested to provide comprehensive evaluation of cardiopulmonary involvement.
Czeyda-Pommersheim F (2015) Amyloidosis: modern cross-sectional imaging. Radiographics 35(5):1381
Erdheim-Chester Disease (ECD)
Definition
Erdheim-Chester disease (ECD) is a rare form of non-Langerhans cell histiocytosis, with systemic infiltration by CD68+ and CD1a− histiocytes without Birbeck granules. Diagnosis in adulthood is a distinctive feature of ECD compared to other forms of non-Langerhans histiocytosis. The clinical manifestation is heterogeneous due to the variability in organ involvement. However, long bones are primarily affected in 95 % of patients. Extra-osseous manifestation is also seen, particularly in the retroperitoneum (perirenal rind of soft tissue), central nervous system (sellar with diabetes insipidus or extra-sellar), and thorax (myocardium, pericardium, lung, mediastinum, pleura, and vessels).
ECD
Campochiaro C (2015) Erdheim-Chester disease. Eur J Intern Med 26(4):223
Zaveri J (2014) More than just Langerhans cell histiocytosis: a radiologic review of histiocytic disorders. Radiographics 34(7):2008
High-Resolution CT: HRCT
Key Signs
Smooth thickening of interlobular septa (►)
Smooth bronchovascular thickening (peribronchial cuffing) (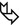 )
)
Smooth subpleural thickening (➨)
Prominence of centrilobular structures
Distribution
Apical, anterior, and peripheral regions of the lungs are predominantly involved by symmetric smooth interlobular reticulation; also patchy or unilateral.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


