Diffuse Malignant Mesothelioma
Valerie W. Rusch
Diffuse malignant pleural mesothelioma (MPM) is an uncommon and lethal cancer for which there are limited treatment options. The first report of a primary pleural tumor, presumably a MPM, is attributed to Lieutaud in 1767, but there was no precise pathologic description until Klemperer and Rabin classified MPMs as either localized or diffuse.52 However, MPM was largely regarded as a medical curiosity until 1960, when Wagner and coworkers reported 33 cases of diffuse MPM in South African asbestos mine workers.116 Subsequent studies confirmed that asbestos exposure was the major risk factor for MPM.97,120 The epidemiology of diffuse MPM is now well defined, but its tumor biology remains poorly understood.
The worldwide incidence of MPM is increasing because of the large number of individuals who were exposed to asbestos from the 1930s to the 1960s in asbestos mines and related industries, before the relationship between asbestos and MPM was recognized. According to the U.S. Cancer Statistics (UCSC) database, approximately 3,035 cases occurred in the United States in 2004.111 In western Europe alone, a quarter of a million deaths are projected over the next 30 years.80 MPM is also increasing in some Asian and Middle Eastern countries, including Japan and Egypt.40,65 It is important for thoracic surgeons to be knowledgeable about MPM because they are often called on to make the diagnosis and recommend treatment.
Epidemiology and Incidence
A relationship between asbestos exposure and interstitial lung disease was first recognized in 1906, when deaths among asbestos textile workers from pneumoconiosis were reported in England and France.9,66 Case reports suggested a link between asbestos exposure and diffuse MPM,112,119 but this was not clearly established until Wagner’s report.116 Wagner was appointed Asbestosis Research Fellow at the South Africa Pneumoconiosis Research Unit in 1954 and was charged with determining if all types of asbestos caused the same diseases. An increasing frequency of a fatal pleural tumor in patients in the asbestos mining region of South Africa led him to study pleural biopsies on these patients. The surprise finding of MPM in these biopsies prompted a careful epidemiologic study. Asbestos exposure was the single factor common to all these cases. Subsequently, Wagner studied this problem further in Great Britain and confirmed the link between asbestos exposure and MPM.115 Later, Musk and colleagues studied 7,000 individuals—at the Wittenoom asbestos mines in Western Australia—who had extensive exposure to asbestos from 1943 to 1966.68 The first case of MPM was diagnosed in 1960. By the end of 1986, a total of 94 cases of MPM, 141 cases of lung cancer, and 356 successful compensation claims for asbestosis were recorded among former miners and their family members. An additional 692 cases of MPM are expected to occur in this cohort between 1987 and 2020. This history at the Wittenoom mines is considered to represent the worst industrial disaster in Australian history.
The type of asbestos fiber plays a critical role in the risk of developing MPM.27 Asbestos is a silicate fiber and includes two mineralogic groups, amphibole (crocidolite, amosite, tremolite, anthrophyllite, and actinolite asbestos) and serpentine (chrysotile asbestos) fibers. Serpentine fibers are large, curly fibers that lodge in the major airways, whereas amphibole fibers are narrow, straight fibers that migrate through the lymphatics of the pulmonary parenchyma and accumulate in the interstitial spaces and the subpleural region. It is the amphibole fibers, especially crocidolite asbestos (“blue asbestos”) that are most clearly associated with MPM. Chrysotile is more closely associated with the development of lung cancer.
Crocidolite asbestos is mined only in South Africa and Western Australia but has been exported all over the world for various industrial uses. Chrysotile (“white asbestos”) accounts for 97% of worldwide asbestos production and is mined principally in the Ural Mountains in Russia, Quebec Province in Canada, Zimbabwe and Swaziland in South Africa, the Italian Alps, and Cyprus. Chrysotile itself is not thought to cause MPM but is often contaminated with amphibole fibers, such as tremolite or amosite (“brown asbestos”). However, controversy over the potential role of chrysotile asbestos in causing MPM persists.64
The areas of the world that have a high incidence of MPM are those with asbestos mines and countries that have shipyards, insulation, construction, and automobile industries that use large amounts of asbestos.49 In North America, the highest-incidence areas include the Canadian provinces of Quebec and British Columbia, and Seattle, Hawaii, San Francisco–Oakland, New York–New Jersey, New Orleans, and Norfolk, Virginia, all of which have large shipyards. It is difficult to document a relationship between the duration or intensity of asbestos exposure and the risk of developing MPM, but patients with peritoneal mesothelioma usually have a history of heavier exposure than do patients with pleural disease. 83
MPM is also caused by other naturally occurring and manufactured silicate fibers that share the physical properties of amphibole asbestos. The most notable example is erionite, a zeolite
fiber found in volcanic deposits in central Turkey, where it is the major material for building homes. Baris11 found that in Karain, Turkey, a village of 604 persons, MPM was the single most common cause of death, with 62 cases recorded from 1970 to 1981. In this village, MPM frequently occurred in individuals who were in their twenties or thirties because they were exposed to erionite from birth.
fiber found in volcanic deposits in central Turkey, where it is the major material for building homes. Baris11 found that in Karain, Turkey, a village of 604 persons, MPM was the single most common cause of death, with 62 cases recorded from 1970 to 1981. In this village, MPM frequently occurred in individuals who were in their twenties or thirties because they were exposed to erionite from birth.
Table 68-1 World Health Organization Histological Classification of Tumors of the Pleura | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
Less common causes of MPM also exist. Radiation exposure at periods ranging from 10 to 31 years before the development of MPM is the most clearly documented of these causes (e.g., individuals who received radiation for Hodgkin’s disease as young adults).57 Extravasation of radioactive thorium dioxide (Thorotrast, a contrast agent previously used for radiologic procedures) and exposure to isoniazid in utero were reported to cause MPM.8 However, in contrast to lung cancer, for which asbestos and smoking act as synergistic carcinogens, smoking is not a risk factor for MPM.
The peak age for the development of MPM is during the sixth decade of life. Because most patients develop MPM as a result of occupational exposure to asbestos, the increased incidence of this disease has occurred in men. In the United States, the incidence in women remains at the baseline level of 3 cases per million population per year, whereas in men it has risen to 15 cases per million. MPM is predominantly a disease of older adults because of the long latency period (at least 20 years) between exposure to causative agents and the development of cancer, but it can occur in childhood.39 In that setting, it is usually idiopathic. MPM sometimes develops in young adults because of exposure to risk factors during childhood.50
Pathology
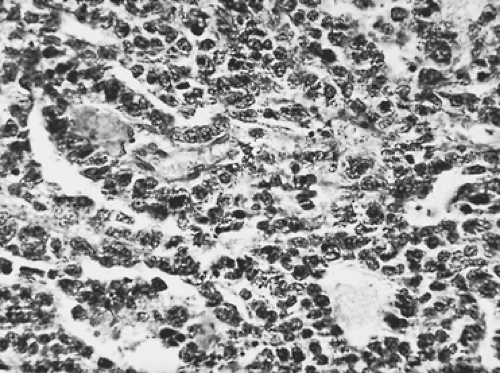
MPMs arise from multipotential mesothelial or subserosal cells that can develop into either an epithelial or a sarcomatoid neoplasm (Table 68-1). In contrast to fibrous tumors of the pleura, diffuse MPMs always have an epithelial component. However, they often exhibit a variable mixture of epithelial and sarcomatoid features (Figs. 68-1 and 68-2). In a review of 819 cases, Hillerdal reported that 50% were of epithelial type, 34% were of mixed type, and 16% were of the sarcomatoid type.46 More
recent series confirm that the pure epithelial tumors are the most common, the mixed or biphasic tumors the second most common, and the pure sarcomatoid tumors the least common histologic subtypes of MPM. MPM can be confused with other neoplasms, especially metastatic adenocarcinoma, when light microscopy is the sole method of diagnosis.43 Very early MPMs can also be difficult to distinguish from benign mesothelial hyperplasia, and the rare desmoplastic form of MPM often resembles benign fibrosis because of its predominantly fibroblastic cell type and sparsely cellular appearance.
recent series confirm that the pure epithelial tumors are the most common, the mixed or biphasic tumors the second most common, and the pure sarcomatoid tumors the least common histologic subtypes of MPM. MPM can be confused with other neoplasms, especially metastatic adenocarcinoma, when light microscopy is the sole method of diagnosis.43 Very early MPMs can also be difficult to distinguish from benign mesothelial hyperplasia, and the rare desmoplastic form of MPM often resembles benign fibrosis because of its predominantly fibroblastic cell type and sparsely cellular appearance.
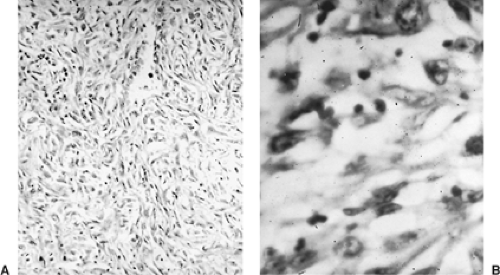 Figure 68-2. Photomicrograph of malignant mesothelioma of the sarcomatous variety. A: Low-power magnification. B: High-power magnification. |
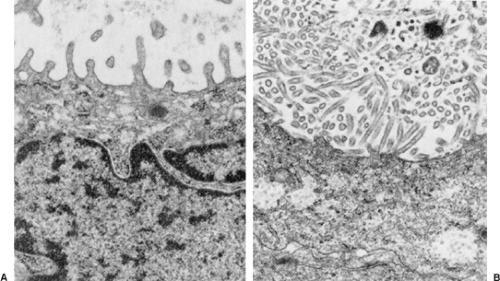
Both histochemical and immunostains help to distinguish MPM from other tumors. Pulmonary adenocarcinomas usually stain positively with mucicarmine, whereas MPM does not. Approximately 20% of epithelial MPMs produce hyaluronic acid, which can be seen either within or between cells with an Alcian blue/colloidal iron stain. MPMs stain positively for low-molecular-weight cytokeratins, a feature that distinguishes them from sarcomas, and for calretinin. They rarely stain for carcinoembryonic antigen, a feature that distinguishes them from adenocarcinomas (Table 68-2). Should immunohistochemical stains yield equivocal results, electron microscopy can provide a definitive diagnosis. MPMs have long, sinuous microvilli, while adenocarcinomas have short, straight microvilli covered by a fuzzy glycocalyx (Fig. 68-3).
Molecular Biology
The sequence of genetic events leading to MPM is not well defined. Asbestos may cause genetic damage either directly or indirectly through the release of cytokines and mutagenic oxygen radicals. It also causes release of tumor necrosis factor-alpha (TNF-α) and its receptor from macrophages and mesothelial cells, which in turns leads to activation of NF-κB, allowing mesothelial cells with DNA damage to divide rather than die.20 Individual genetic susceptibility may also play a role in MPM development, as may the immunosuppressive effects of asbestos.70
Table 68-2 Mesothelioma Versus Adenocarcinoma | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||
Like other solid tumors, MPM is characterized biologically by the presence of multiple cytogenetic and molecular abnormalities. Deletion of regions in the short arms of chromosomes 1, 3, and 9 and the long arms of chromosomes 6, 13, 15, and 22 are commonly seen.67 The most frequent molecular abnormality is the loss of CDKN2A/ARF, which encodes for the tumor suppressor genes p16INK4a and p14ARF. Loss of these genes adversely affects the Rb and p53 pathway. NF2 (Merlin) is also frequently mutated or dysregulated in MPM.82
Frequent mutations of the Wilms’ tumor1 gene (WT1) have been identified both in primary MPM and in cell lines.54 WT1 encodes a transcription factor which regulates the early growth response 1 (EGR1), the insulin growth factor 1 receptor (IGF-1R) and the epidermal growth factor receptor (EGFR) genes and is thought to be important in mediating apoptosis. The
AKT pathway, which antagonizes cell cycle arrest, is frequently activated in MPM.
AKT pathway, which antagonizes cell cycle arrest, is frequently activated in MPM.
Multiple growth factors and their receptors—including transforming growth factor-alpha (TGF-α), epidermal growth factor receptor (EGFR), platelet-derived growth factor (PDGF), insulin-like growth factor (IGF), hepatocyte growth factor (HGF)—are overexpressed in MPM or in mesothelial cell lines.82 Ohta74 reported that the vascular endothelial growth factors (VEGF and VEGF-C) and their receptors, which regulate angiogenesis, were overexpressed in at least 50% of MPM tumors and cell lines.73 The same group of investigators found that thrombospondin-1 (TSP-1), which also regulates angiogenesis, was overexpressed in 95% of MPM tumors examined, although this did not appear to correlate with prognosis.
There is considerable controversy about whether simian virus 40 (SV40) is present in MPM and its potential role in carcinogenesis. SV40 sequences have been identified in MPM specimens from the United States but not from several other countries, including Finland.47,110 It is hypothesized that some individuals may have been exposed to SV40 through contaminated polio vaccines used during the 1950s. SV40 is well known to be tumorigenic in animal models, potentially through inactivation of the p53 and Rb tumor suppressor genes, and it has been postulated that SV40 may function as a cocarcinogen, leading to dysregulation of one or more genetic pathways. However, a more recent study suggests that SV40 is not truly present in MPM and may only have been a contaminant in previous studies.60
In summary, the biology of MPM is complex and reflects the interplay of multiple genetic abnormalities, most frequently the deletion or inactivation of various tumor suppressor genes.59 These abnormalities may arise from a multifactorial impact of asbestos fibers on cell cycle regulation and on cellular and immunologic function. Further investigation is needed to identify the precise progression of molecular events that lead to MPM and govern its clinical behavior.
Clinical Presentation
The clinical presentation of diffuse MPM is insidious and nonspecific. MPM has traditionally been portrayed as a diffuse, massive tumor that causes excruciating chest pain, but these signs and symptoms are seen only when MPM reaches a locally advanced stage. During the early stages of disease, dyspnea is the predominant symptom and is related to the presence of an effusion. When the effusion is drained, patients are asymptomatic. As the tumor grows, patients develop ill-defined, mild, but continuous chest discomfort. Dyspnea may actually improve during this phase of the disease because, with tumor growth, the pleural surfaces fuse and the effusion resolves. Only when the disease progresses does the patient develop severe chest pain, which is related to tumor infiltration of the chest wall and intercostal nerves, and worsening dyspnea caused by tumor entrapment of the lung. In the final stages of disease, the dyspnea and chest pain become severe and unremitting. These symptoms are caused by encasement of the chest wall, lung, and mediastinum, occasionally associated with mediastinal shift and compression of the contralateral lung. The tumor may also extend directly through the pericardium, causing a pericardial effusion or myocardial metastases. The symptoms of locally advanced pleural disease may also be compounded by the development of ascites from direct extension of the tumor through the diaphragm or a contralateral pleural effusion from metastatic disease. Weight loss occurs in approximately 30% of patients but is seen only in the advanced stages of the disease. Uncommon symptoms include cough, weakness, anorexia, fever, hemoptysis, hoarseness, dysphagia, and Horner’s syndrome. Spontaneous pneumothorax is a rare presenting symptom.98
In the early stages of disease, the findings on physical examination are nonspecific. A pleural effusion may cause dullness to percussion and decreased breath sounds, but the chest examination is otherwise normal. In the late stages of disease when tumor encases the hemithorax, the excursion of the chest with respiration diminishes and the chest wall is noticeably contracted. Diffuse dullness to percussion and decreased breath sounds are present over the entire hemithorax and there is a subtle fullness of the intercostal spaces. If the tumor has grown through the intercostal spaces or has implanted in the site of a previous thoracentesis or thoracotomy incision, palpable soft tissue masses may be found in the chest wall. Palpable supraclavicular or axillary nodes or ascites may be present if the tumor has metastasized.
Paraneoplastic syndromes are uncommon, but autoimmune hemolytic anemia, hypercalcemia, hypoglycemia, the syndrome of inappropriate secretion of antidiuretic hormone, and hypercoagulability not related to thrombocytosis have been reported.89 Thrombocytosis (platelet count of 400,000/mL or greater), occurs in approximately 30% to 40% of patients. This is sometimes associated with a leukemoid reaction but does not seem to increase the risk of thromboembolic episodes.75
No tumor markers are yet routinely used for MPM. Serum hyaluronan may be elevated in some patients.26 Hyaluronan can be measured using a commercial kit, but the kit is not readily available in most hospitals. CA-125 is elevated in only 20% of patients, so its use as a serum marker is limited.93 Recently serum osteopontin has been reported to distinguish persons with asbestos exposure who do not have cancer from those who have MPM.77 In 48 patients, soluble mesothelin-related protein (SMRP) levels were reported to parallel the clinical course of MPM.88 Larger studies are needed to validate these encouraging initial findings.
Radiologic Features
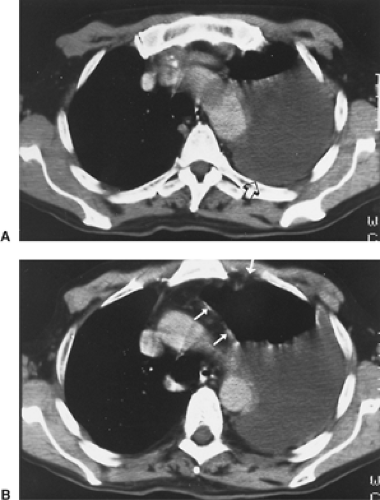
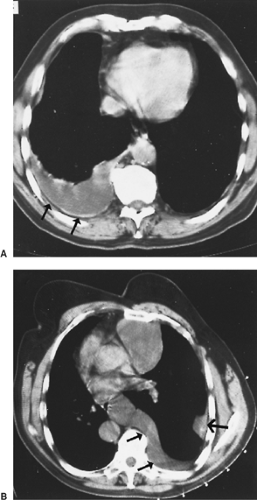
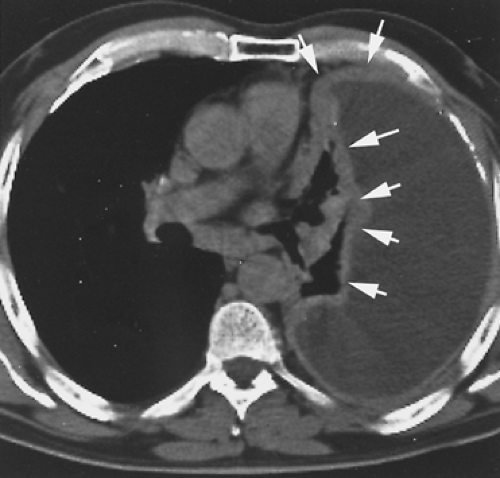
The radiographic appearance of MPM is variable and is related to the stage of the tumor at diagnosis. In early-stage MPM, a large pleural effusion is often the only sign of disease (Fig. 68-4). Subtle pleural thickening or small, discrete, pleural-based masses may be seen on computed tomography (CT) (Fig. 68-5). Subsequently, larger pleural-based masses become evident and are often intermixed with multiloculated effusions. A dominant pleural-based mass may be the initial presentation, but ultimately the involvement of the pleura is always diffuse. Eventually, a thick confluent pleural rind develops, with encasement of the lung and obliteration of the pleural space (Fig. 68-6). Mediastinal adenopathy, direct extension of the tumor into the mediastinum, involvement of the pericardium with pericardial effusion, and extension into the chest wall or through the diaphragm are seen with locally advanced disease (Fig. 68-7). CT permits a far better assessment of the extent of the disease than standard radiography and is the standard way to stage patients as well as to assess response to treatment and detect recurrent disease postoperatively. However, it is inaccurate in diagnosing
chest wall involvement or extension through the diaphragm.90 It was hoped that magnetic resonance imaging (MRI) would prove more accurate in this regard, but a prospective study comparing CT and MRI for preoperative staging showed that MRI is not significantly better than CT in defining the local extent of tumor.44 Therefore CT remains the standard imaging study.
chest wall involvement or extension through the diaphragm.90 It was hoped that magnetic resonance imaging (MRI) would prove more accurate in this regard, but a prospective study comparing CT and MRI for preoperative staging showed that MRI is not significantly better than CT in defining the local extent of tumor.44 Therefore CT remains the standard imaging study.
Pass78 studied the relationship of preoperative tumor volume by three-dimensional CT reconstructions on outcome in patients undergoing resection for pleural MPM. In 48 patients, the median survival for a preoperative tumor volume less than 100 mL was 22 months, versus 11 months for a volume greater than 100 mL (p = 0.03). Progressively higher tumor stage was associated with higher median preoperative tumor volume, which in turn was associated with a greater likelihood of lymph node metastasis.78
Recently, the utility of fluorodeoxyglucose positron emission tomography (FDG PET) scanning in the initial staging and assessment of treatment response has been established. PET identifies metastatic disease in approximately 10% of patients considered to have resectable disease by CT scan. The PET standard uptake value (SUV) correlates with the presence of lymph node metastases and is prognostic for overall survival. PET SUV is also a sensitive indicator of response to chemotherapy.21,35,100 Therefore PET is now routinely used for the initial staging of MPM and to monitor treatment.
Diagnosis
Because most patients present with a pleural effusion, a thoracentesis is usually the initial diagnostic procedure, but pleural fluid cytology identifies malignancy in less than 50% of cases. Thoracoscopy is the optimal diagnostic procedure because it yields a definitive diagnosis without committing the patient to a
major surgical procedure. The appearance of the pleural space is variable. In the earliest stage of MPM, involvement of the pleura is microscopic and the only visible finding is a large pleural effusion. As the disease progresses, the thoracoscopic appearance evolves from tumor studding of the parietal pleura with a free pleural space and a large pleural effusion to studding of both parietal and visceral pleurae. The next stage involves larger but still discrete masses with multiloculated pleural effusions and, finally, a confluent irregular sheet of tumor with obliteration of the pleural space. The tumor ranges from soft, friable, and hypervascular to densely fibrotic, depending on the mixture of cell types. No clinical findings are pathognomonic of MPM.
major surgical procedure. The appearance of the pleural space is variable. In the earliest stage of MPM, involvement of the pleura is microscopic and the only visible finding is a large pleural effusion. As the disease progresses, the thoracoscopic appearance evolves from tumor studding of the parietal pleura with a free pleural space and a large pleural effusion to studding of both parietal and visceral pleurae. The next stage involves larger but still discrete masses with multiloculated pleural effusions and, finally, a confluent irregular sheet of tumor with obliteration of the pleural space. The tumor ranges from soft, friable, and hypervascular to densely fibrotic, depending on the mixture of cell types. No clinical findings are pathognomonic of MPM.
When thoracoscopy is not technically feasible because the pleural space is obliterated by locally advanced tumor, the small incision made for thoracoscopy is extended to a length of 6 cm and is used for open pleural biopsy by resection of a short segment of the overlying rib. Because MPM easily implants in the chest wall, the incision should be placed in line with a possible subsequent thoracotomy incision so that it can be excised or reutilized at the time of the definitive operation. Exploratory thoracotomy should be avoided because it exposes patients who have metastatic adenocarcinoma to the unnecessary morbidity of a major operation and complicates definitive surgical resection in patients with MPM.
CT scan of the chest and upper abdomen and FDG PET scan are the routine initial staging studies. Bronchoscopy is not required because MPM patients do not have endobronchial tumor. The role of mediastinoscopy in the management of MPM is controversial. While it identifies some patients with mediastinal nodal metastases (N2 disease), approximately 25% of the mediastinal nodes to which MPM metastasizes are inaccessible to mediastinoscopy. Because it is a poor prognostic factor in MPM,95,103,107 some investigators choose not to operate at all on patients with N2 disease, while others use mediastinoscopy to select patients for preoperative chemotherapy.81 Further studies are needed to establish a standard of care in this regard.95,96 “Extended” surgical staging including mediastinoscopy and laparoscopy has been advocated by some investigators,84 but this is not generally accepted. Laparoscopy is appropriate to determine whether transdiaphragmatic tumor extension seen on imaging studies is truly present.24
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree