INDICATIONS/CONTRAINDICATIONS
Injury to the cervical spine above the level of C1 to C2 results in quadriplegia and prevents stimulation of the diaphragm. It is important to realize that these patients have intact phrenic nerves but are simply unable to transmit a nerve impulse to the diaphragm. Every year, there are approximately 12,000 new patients affected with SCI. More than 50% of this group will develop quadriplegia with >4% requiring mechanical ventilation. The medical cost of a mechanically ventilated patient can approach $200,000 a year. In addition to this astronomical cost, patients with mechanical ventilation have a much poorer quality of life and are riddled with complications. Ventilated patients can experience difficulty with speech, inability to eat properly leading to frequent aspirations, increased production of secretions requiring frequent suctioning, and an increase rate of ventilator-associated pneumonias. It has been reported that the estimated life expectancy for a 20 year old with SCI requiring mechanical ventilation, is more than 41 years shorter than a person of the same age who has a SCI that does not require mechanical ventilation. Diaphragm pacers are placed in this cohort of patient with the goal of decreasing ventilator dependence.
Patients with central alveolar hypoventilation do not have the normal increased respiratory response when hypoxic or hypercapneic. The diminished response is present night and day; however, the patient is able to make a conscious effort to breathe during the day, which is not the case at night. This form of hypoventilation can be congenital or acquired. The congenital form affects 1 in 50,000 live births. The diagnosis of congenital central hypoventilation syndrome requires documentation of hypoventilation during sleep in the absence of primary respiratory, cardiac, or neuromuscular disease or a brainstem lesion. Once, it is diagnosed, children require nighttime positive pressure ventilation for the rest of their lives. The acquired form can be secondary to brainstem stroke, surgical trauma, tumor, hemorrhage, or meningoencephalitis. Implantation of a diaphragmatic pacer in these patients, whether a child or adult, can drastically improve the patient’s quality of life by releasing them from a lifelong requirement of nightly positive pressure ventilation. It is important to differentiate between central alveolar hypoventilation and obstructive sleep apnea as the latter does not benefit from implantation of a diaphragmatic pacer.
Contrary to initial thoughts, some recent studies have reported utility of diaphragmatic pacing in the amyotropic lateral sclerosis (ALS or Lou Gehrig disease). ALS patients have an idiopathic motor neuron degeneration in the cerebral cortex, brainstem, and spinal cord. This is a progressive and ultimately fatal disease. All of the muscles utilized for respiration are adversely affected in ALS patients resulting in progressive respiratory failure requiring mechanical ventilation. More than 80% of deaths in ALS are attributed to pulmonary failure and complications. It was conceptualized that in these patients, the utilization of diaphragm pacing before the onset of respiratory failure may help maintain diaphragm strength and provide trophic effects allowing the phrenic nerve neurons to remain viable much longer. The goal with diaphragm pacing would be to increase the time from diagnosis to the onset of respiratory failure requiring mechanical ventilation in ALS patients.
 PREOPERATIVE PLANNING
PREOPERATIVE PLANNING
Diaphragmatic pacing has been proven successful in removing ventilator dependency in a highly specific subset of patients. It is critical to select the appropriate patients for this procedure. Only patients who have an intact phrenic nerve and functional diaphragm should be selected for implantation. Patients with SCI have had partial and sometimes full recovery of the phrenic nerve up to 12 months after the initial injury. Therefore, it is important to wait 12 months before assessing these patients for a diaphragm pacer. Once phrenic nerve and diaphragmatic function is confirmed the patient can be considered for surgical placement of a diaphragm pacer.
Standard preoperative workup includes:
 History and physical examination
History and physical examination
 Particular attention needs to be paid to signs and symptoms of respiratory and neurologic deficits. Patients with underlying intrinsic lung disease may not benefit from diaphragmatic pacing because severe lung pathology may be the major contributor to poor oxygenation and ventilation. In which case, a pacer may not have a dramatic impact on the patient’s respiratory status. CT scans of the brain, cervical spine, neck, and chest are important to rule out symptoms secondary to a mass lesion.
Particular attention needs to be paid to signs and symptoms of respiratory and neurologic deficits. Patients with underlying intrinsic lung disease may not benefit from diaphragmatic pacing because severe lung pathology may be the major contributor to poor oxygenation and ventilation. In which case, a pacer may not have a dramatic impact on the patient’s respiratory status. CT scans of the brain, cervical spine, neck, and chest are important to rule out symptoms secondary to a mass lesion.
 Pulmonary function tests (PFTs)
Pulmonary function tests (PFTs)
 In patients with a paralyzed diaphragm, a restrictive process is seen on PFTs. There is a loss of vital capacity, which is worse when measured in the supine position.
In patients with a paralyzed diaphragm, a restrictive process is seen on PFTs. There is a loss of vital capacity, which is worse when measured in the supine position.
 It is important to look for evidence of intrinsic lung disease to better select patients who will benefit from pacing.
It is important to look for evidence of intrinsic lung disease to better select patients who will benefit from pacing.
 Chest x-ray (CXR)
Chest x-ray (CXR)
 A CXR is more useful in identifying patients with unilateral diaphragmatic paralysis as it will show an elevated hemidiaphragm on the affected side compared to the normal diaphragm on CXR. Patients with bilateral diaphragmatic paresis may not have an obvious finding on CXR as both hemidiaphragms may elevate and thus appear in “normal” position.
A CXR is more useful in identifying patients with unilateral diaphragmatic paralysis as it will show an elevated hemidiaphragm on the affected side compared to the normal diaphragm on CXR. Patients with bilateral diaphragmatic paresis may not have an obvious finding on CXR as both hemidiaphragms may elevate and thus appear in “normal” position.
 Fluoroscopic “sniff test”
Fluoroscopic “sniff test”
 When a patient takes a deep breath, intercostal and accessory muscles are the main contributors to the respiratory excursion. Breathing through one’s nose, “sniffing,” ensures diaphragmatic involvement. A patient is asked to sniff while in the supine position. Radiopaque markers are used to measure maximal diaphragmatic movement. In a patient with normal phrenic nerves, the sniff will result in a quick downward deflection of the diaphragm. The test is positive for diaphragmatic paralysis if there is paradoxical upward movement of the diaphragm during inspiration.
When a patient takes a deep breath, intercostal and accessory muscles are the main contributors to the respiratory excursion. Breathing through one’s nose, “sniffing,” ensures diaphragmatic involvement. A patient is asked to sniff while in the supine position. Radiopaque markers are used to measure maximal diaphragmatic movement. In a patient with normal phrenic nerves, the sniff will result in a quick downward deflection of the diaphragm. The test is positive for diaphragmatic paralysis if there is paradoxical upward movement of the diaphragm during inspiration.
 Percutaneous cervical electrical stimulation
Percutaneous cervical electrical stimulation
 This is the gold standard for testing phrenic nerve function. Electrodes are placed in the neck and electrical stimulation is performed. And intact phrenic nerve results in hemidiaphragm stimulation and contraction.
This is the gold standard for testing phrenic nerve function. Electrodes are placed in the neck and electrical stimulation is performed. And intact phrenic nerve results in hemidiaphragm stimulation and contraction.
 Prolonged latency or failure to conduct indicates poor phrenic nerve conduction.
Prolonged latency or failure to conduct indicates poor phrenic nerve conduction.
 Social assessment
Social assessment
 Patients undergoing diaphragmatic pacemakers need to be highly motivated with a solid social and economic foundation. It is vital that the caregivers are enthusiastic as well since postoperative manipulations of the pacer may require frequent visits with the medical team.
Patients undergoing diaphragmatic pacemakers need to be highly motivated with a solid social and economic foundation. It is vital that the caregivers are enthusiastic as well since postoperative manipulations of the pacer may require frequent visits with the medical team.
Of special note, patients with congenital central hypoventilation syndrome as well as patients with high cervical SCI, have been reported to have bradyarrhythmias requiring cardiac pacing systems. Diaphragmatic pacers do not have sensing capabilities so there is no risk of interference from a cardiac pacemaker. However, there is the potential for a cardiac pacemaker to be influenced by the diaphragmatic pacemaker. In patients with cardiac pacemakers, it is important to discuss the case with the cardiac electrophysiology service as they may need to adjust the cardiac pacemaker settings prior to implantation of a diaphragm pacer. Although this is a theoretical concern, Onder et al. published a report on 20 patients with cardiac pacemakers in whom a diaphragmatic pacer was placed. None of the patients experienced immediate or long-term device-to-device interactions.
Amyotropic Lateral Sclerosis
The preoperative forced vital capacity (FVC) must be greater than 40% in ALS patients otherwise it is been found that there is a high risk of failure to extubate the patient at the conclusion of the implantation procedure.
The American Academy of Neurology recommends that ALS patients with respiratory symptoms and a FVC of less than 50% should be offered noninvasive positive pressure ventilation (NIPPV). Patients considered for diaphragm pacing should have these masks fitted and utilized prior to surgery so they are accustomed to them if they are needed in the immediate postoperative period.
 SURGERY
SURGERY
Currently there are four devices that are available worldwide: Vienna Phrenic Pacemaker (Medimplant, Vienna, Austria), Astrostim (Atrotech Ltd., Tampere, Finland), the Avery Mark IV Phrenic Pacemaker (Avery biomedical, Commack, NY, USA), and the NeuRx Diaphragm Pacing System (DPS: Synapse Biomedical Inc., Oberlin, OH, USA). The first three systems utilize direct phrenic nerve stimulation by directly implanting the electrodes on the phrenic nerve. The NeuRx system places the stimulating electrode directly onto the under surface of the diaphragm. All systems then require connection of the stimulating electrode to a receiver usually placed in a subcutaneous pocket.
In the 1980s it was shown that direct diaphragmatic stimulation can be achieved. Mortimer et al. were able to produce diaphragmatic contractions when they stimulated areas where the phrenic nerve enters the diaphragm. Electrodes placed in this area, known as motor points, were able to produce diaphragmatic contractions similar to those obtained with direct phrenic nerve stimulation. Onders has since published extensively on the effectiveness of laparoscopic electrode placement at motor points in patient with respiratory insufficiency.
The remainder of this chapter will discuss the Avery Mark IV and NeuRx systems.
Components
 Avery Mark IV
Avery Mark IV
 Electrodes surgically placed on bilateral phrenic nerves
Electrodes surgically placed on bilateral phrenic nerves
 Lead wires that connect electrodes to subcutaneous receivers
Lead wires that connect electrodes to subcutaneous receivers
 Antennae that are taped over the receivers
Antennae that are taped over the receivers
 External transmitter
External transmitter
 NeuRx
NeuRx
 Electrodes surgically placed at motor points on bilateral diaphragms
Electrodes surgically placed at motor points on bilateral diaphragms
 Grounding electrode
Grounding electrode
 Control unit
Control unit
 Cable and external battery-powered pulse generator
Cable and external battery-powered pulse generator
Anesthetic Considerations
 Overall strategy should be to avoid paralytics since motor points need to be identified during the surgery.
Overall strategy should be to avoid paralytics since motor points need to be identified during the surgery.
 Rapid reversible, short-acting anesthesia is preferred.
Rapid reversible, short-acting anesthesia is preferred.
 In ALS patients, succinylcholine is contraindicated because it can trigger hyperkalemia in these patients who have denervated muscles with increased acetylcholine receptors.
In ALS patients, succinylcholine is contraindicated because it can trigger hyperkalemia in these patients who have denervated muscles with increased acetylcholine receptors.
 Local anesthesia should be used in all incisions to decrease pain response and minimize the amount of general anesthesia required.
Local anesthesia should be used in all incisions to decrease pain response and minimize the amount of general anesthesia required.
Surgical Procedure
The implantation of phrenic nerve electrodes used for diaphragmatic pacing can be performed through a cervical, thoracic, abdominal approach.
All of these approaches first require an understanding of the path and course taken by the right and left phrenic nerves as they exit the spinal cord and travel to the diaphragm.
While the phrenic nerve contains motor and sensory fibers, the right and left phrenic nerves provide the only motor innervation of the diaphragm. In addition, they supply sensation to the central tendon of the diaphragm. They originate in the neck from C3 to C5 with most of the fibers of the phrenic nerve primarily originating from the fourth cervical nerve. See Figure 20.1.
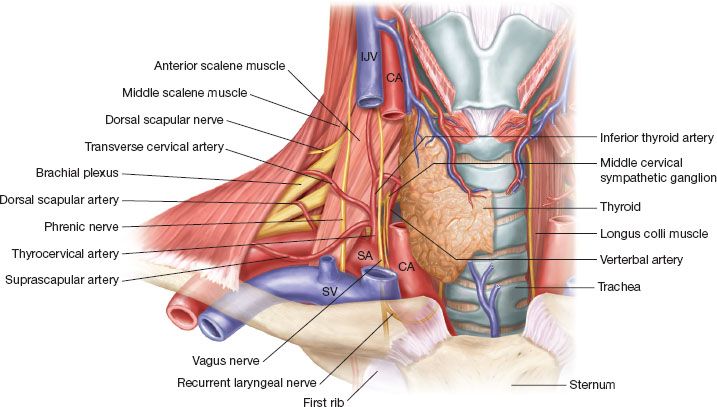
Figure 20.1 Course of phrenic nerve in neck: Note the phrenic nerve traveling on the anterior surface of the anterior scalene muscle as it travels from lateral to medial. The nerve is deep to the transverse cervical artery and the suprascapular artery which can be injured in the dissection. Note the nerve entering the thoracic inlet just lateral to the junction of the internal jugular vein and the subclavian vein.
Right Phrenic Nerve
 After leaving the vertebral foramen from C3 to C5, the right phrenic nerve is identified on the posterolateral aspect of the internal jugular vein.
After leaving the vertebral foramen from C3 to C5, the right phrenic nerve is identified on the posterolateral aspect of the internal jugular vein.
 The nerve exits between the middle scalene muscle posteriorly and the anterior scalene muscle anteriorly to travel obliquely across the anterior surface of the anterior scalene muscle.
The nerve exits between the middle scalene muscle posteriorly and the anterior scalene muscle anteriorly to travel obliquely across the anterior surface of the anterior scalene muscle.
 At this level on the anterior scalene muscle, the phrenic nerve is deep to the prevertebral layer of the deep cervical fascia as well as the transverse cervical artery and the suprascapular artery. As it descends and reaches the inferior and medial aspect of the anterior scalene muscle, the phrenic nerve is superficial to the second portion of the right subclavian artery and as the nerve passes medially it is deep to the right subclavian vein.
At this level on the anterior scalene muscle, the phrenic nerve is deep to the prevertebral layer of the deep cervical fascia as well as the transverse cervical artery and the suprascapular artery. As it descends and reaches the inferior and medial aspect of the anterior scalene muscle, the phrenic nerve is superficial to the second portion of the right subclavian artery and as the nerve passes medially it is deep to the right subclavian vein.
 The nerve then passes deep to the under surface of the first rib and at the level of the first costochrondral junction it will cross the innominate artery.
The nerve then passes deep to the under surface of the first rib and at the level of the first costochrondral junction it will cross the innominate artery.
 As it enters the right hemithorax, the phrenic nerve is found anterior to the superior vena cava and pulmonary hilum running along the pericardium lateral to the right atrium.
As it enters the right hemithorax, the phrenic nerve is found anterior to the superior vena cava and pulmonary hilum running along the pericardium lateral to the right atrium.
 The right phrenic nerve leaves the right hemithorax by passing through the vena caval hiatus in the diaphragm at the level of T8.
The right phrenic nerve leaves the right hemithorax by passing through the vena caval hiatus in the diaphragm at the level of T8.
 The right phrenic nerve then enters the diaphragm through the tendinous portion of the diaphragm just lateral to the inferior vena caval foramen.
The right phrenic nerve then enters the diaphragm through the tendinous portion of the diaphragm just lateral to the inferior vena caval foramen.
 Upon entering the diaphragm the right phrenic nerve will break into three branches on the inferior undersurface of the diaphragm forming an anterior branch, a lateral branch, and a posterior branch.
Upon entering the diaphragm the right phrenic nerve will break into three branches on the inferior undersurface of the diaphragm forming an anterior branch, a lateral branch, and a posterior branch.
 These branches will then spread out in a radial pattern to supply motor function to the right hemidiaphragm.
These branches will then spread out in a radial pattern to supply motor function to the right hemidiaphragm.
Left Phrenic Nerve
 The left phrenic nerve course through the neck is a mirror image of its right-sided counterpart. As the left phrenic nerve enters the left hemithorax, it passes superficial from lateral to medial on the arch of the aorta.
The left phrenic nerve course through the neck is a mirror image of its right-sided counterpart. As the left phrenic nerve enters the left hemithorax, it passes superficial from lateral to medial on the arch of the aorta.
 The left phrenic nerve then passes along the pericardium anterior to the left pulmonary hilum.
The left phrenic nerve then passes along the pericardium anterior to the left pulmonary hilum.
 The left phrenic nerve then curves anteriorly.
The left phrenic nerve then curves anteriorly.
 It will enter the diaphragm anterior to the central tendon and just lateral to the pericardium.
It will enter the diaphragm anterior to the central tendon and just lateral to the pericardium.
 Upon entering the diaphragm the left phrenic nerve will break into three branches on the inferior undersurface of the diaphragm forming an anterior branch, a lateral branch, and a posterior branch.
Upon entering the diaphragm the left phrenic nerve will break into three branches on the inferior undersurface of the diaphragm forming an anterior branch, a lateral branch, and a posterior branch.
 These branches will then spread out in a radial pattern to supply motor function to the left hemidiaphragm.
These branches will then spread out in a radial pattern to supply motor function to the left hemidiaphragm.
Cervical Approach Technique
The cervical approach is usually considered a minimally invasive procedure since it does not require a formal thoracotomy, thoracoscopy, or laparoscopy and is frequently performed as an outpatient procedure. This procedure can be performed under general anesthesia or using local anesthesia with intravenous sedation.
 The patient is placed on the operating room table with a small roll under the patient’s shoulders to aid in the cervical visualization.
The patient is placed on the operating room table with a small roll under the patient’s shoulders to aid in the cervical visualization.
 The patient is prepped and draped in the usual sterile fashion.
The patient is prepped and draped in the usual sterile fashion.
 A 3- to 5-cm incision is made approximately 2 cm above and parallel to the midportion of the clavicle.
A 3- to 5-cm incision is made approximately 2 cm above and parallel to the midportion of the clavicle.
 The platysma can then be divided and the sternocleidomastoid muscle dissected and reflected medially providing exposure of the prescalene fat pad laterally.
The platysma can then be divided and the sternocleidomastoid muscle dissected and reflected medially providing exposure of the prescalene fat pad laterally.
 The prevertebral layer of the deep cervical fascia can be incised exposing the anterior surface of the anterior scalene muscle and internal jugular vein.
The prevertebral layer of the deep cervical fascia can be incised exposing the anterior surface of the anterior scalene muscle and internal jugular vein.
 The phrenic nerve is identified running superficial on the anterior surface of the anterior scalene muscle.
The phrenic nerve is identified running superficial on the anterior surface of the anterior scalene muscle.
 If there is any question as to the identity of the phrenic nerve, a nerve test probe can be used to test and identify the nerve.
If there is any question as to the identity of the phrenic nerve, a nerve test probe can be used to test and identify the nerve.
 Meticulous dissection is used to free a portion of the phrenic nerve taking care to avoid electrical injury to the nerve.
Meticulous dissection is used to free a portion of the phrenic nerve taking care to avoid electrical injury to the nerve.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


