27

Diagnostic Lung Cytology
Normal Lung Cytology
The oropharynx and hypopharynx are lined by stratified, nonkeratinizing squamous epithelium. The nasopharynx and larynx are lined partly by stratified nonkeratinizing epithelium and partly by respiratory type columnar epithelium. The epithelial lining of the tracheobronchial tree, down to the level of the bronchioles, is pseudostratified, ciliated, mucus-secreting columnar (respiratory) epithelium. Mucus cells disappear, ciliated epithelial cells decrease, and Clara cells increase as the terminal bronchiole is approached. Major cell types present in airway epithelium include basal (reserve), goblet (mucus secreting), ciliated columnar, neuroendocrine (Kulchitsky), and reactive squamous cells. Clara cells are found in bronchioles.1 Other airway cell types include serous, brush, and intermediate.
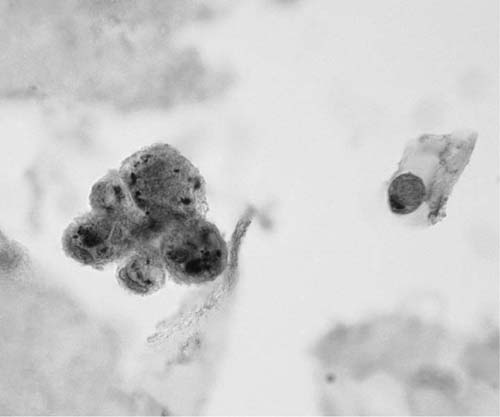
Mature nucleate squamous cells with abundant, eosinophilic cytoplasm and small pyknotic nuclei, similar to superficial squamous cells found in smears from the uterine cervix, exfoliate from the nonkeratinizing squamous epithelium of the oropharynx, are usually found in sputum specimens, and are often the major cell type present. They should be differentiated from the smaller metaplastic squamous cells, resembling metaplastic parabasal type squamous cells of the uterine cervix, that may be found in sputum presumably in response to irritation of the lower respiratory tract (Fig. 27–1).
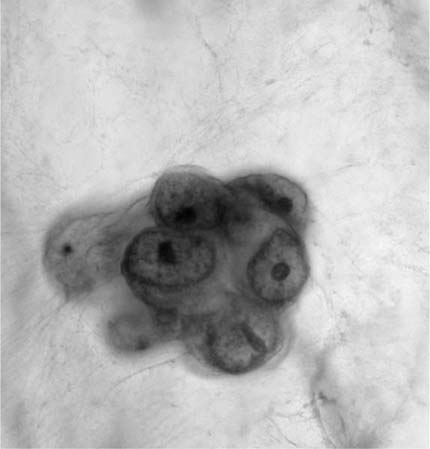
Basal (reserve) cells are uncommonly encountered in sputum and other types of lung cytologic material. They are small cells, usually in tightly cohesive clusters, with round to oval nuclei, small nucleoli, and scant cytoplasm. Tight intercellular cohesion serves to differentiate basal cells from potential “look-alikes” such as cells shed from small cell carcinomas, carcinoids, lymphomas, and lymphocytes.
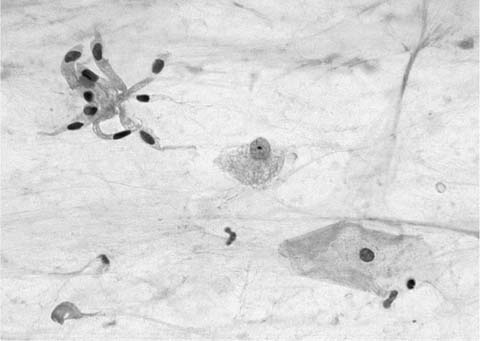
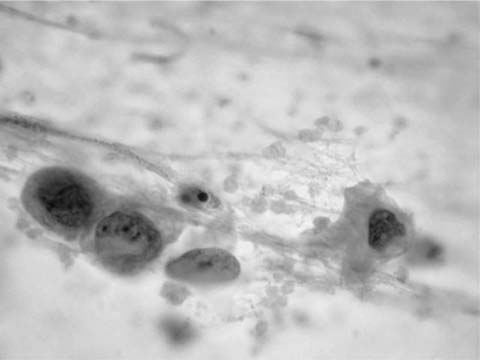
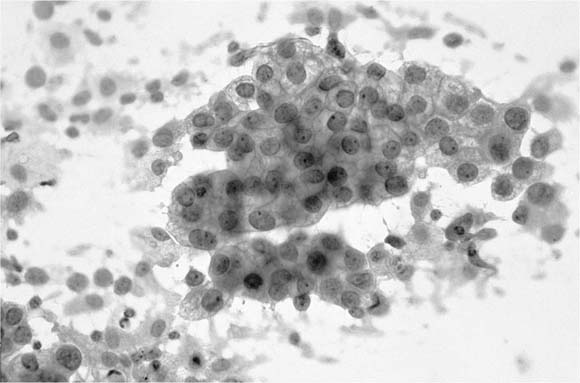
Ciliated columnar cells are not commonly found in sputum, except following lung instrumentation, after prolonged bouts of coughing, or as a contaminant from the nasopharynx. They are the commonest cell type in bronchial brush and wash specimens, are often found in lung fine-needle aspirates, and can contaminate bronchioloalveolar lavage (BAL) specimens (Fig. 27–2). The borders of the fragile columnar cell cytoplasm blend with the lateral borders of the nucleus when the cell is viewed longitudinally. A thick terminal bar at the luminal surface of the cell supports cilia. Columnar cells form a “honeycomb” with centrally placed nuclei when a cell cluster is viewed in cross section. Cilia-bearing cytoplasm may become detached from the remainder of the cell, a process termed ciliocytophoria. Mucus-secreting goblet cells, like ciliated cells, are uncommon in sputum and common in bronchial brush and wash specimens. Goblet cell nuclei are in the base of columnar, vacuolated cytoplasm.
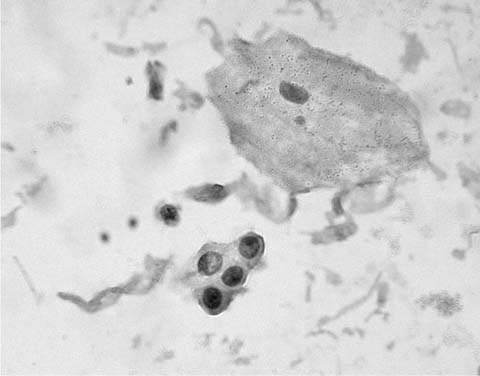
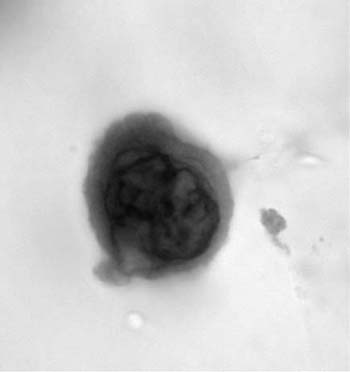
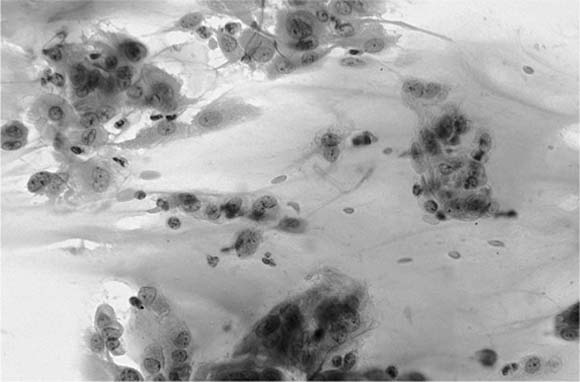
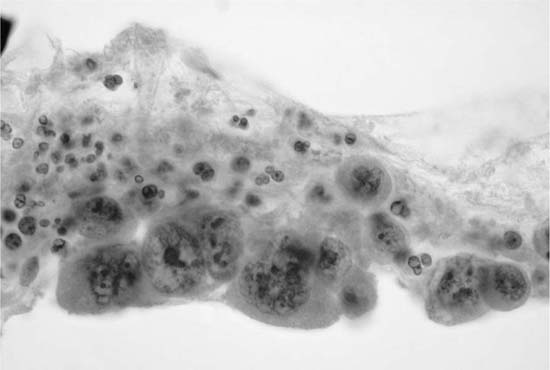
Alveolar macrophages (“dust cells”) (Fig. 27–3) are the hallmark of deep cough sputum specimens. No or few alveolar macrophages in sputum imply that the specimen is saliva or comes from the oropharynx and is unsatisfactory for cytologic examination. Macrophage cytoplasm is variable; it can be opaque to finely vacuolated, often contains dust or pigment, and has a well-defined rim. Nuclei are eccentric, often bean shaped, with fine to coarse chromatin and inconspicuous nucleoli. Macrophages are usually single and, if in clusters, are loosely associated rather than cohesive. The single cell distribution and lack of intercellular cohesion are cytologic features aiding in the differentiation of macrophages from adenocarcinoma cells, which tend to occur in cohesive clusters.
FIGURE 27–1 Metaplastic squamous cells (sputum, Pap stain).
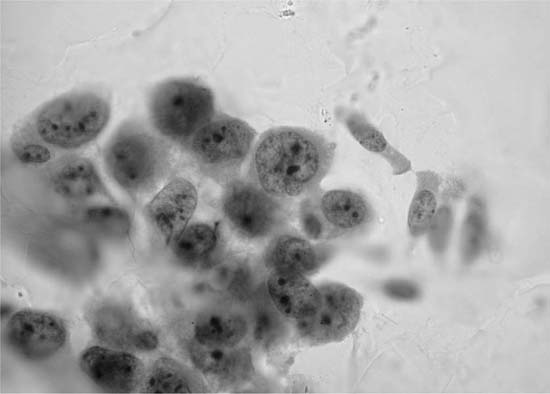
FIGURE 27–2 Normal bronchial epithelial cells, macrophages, and squamous cell (sputum, Pap stain).
FIGURE 27–3 Alveolar macrophages, dust cells (sputum, Pap stain).
Neutrophils, lymphocytes, and other inflammatory cells are common in normal and abnormal pulmonary cytologic material of all types. Large numbers of neutrophils are indicative of bacterial pneumonia. A large number of lymphocytes may be associated with pulmonary lymphoproliferative disorders.
Bronchiolar epithelium may be found in bronchial brushes and lung fine-needle aspirates as cohesive sheets of cells with bland, round, uniform nuclei centrally placed in amphophilic cytoplasm. Typically bronchiolar epithelial cell sheets are few and small. Abundant cellular material should raise the possibility of bronchioloalveolar carcinoma.
Alveolar type 1 and type 2 pneumocytes are probably not identifiable in standard lung cytologic material. Reactive type 2 pneumocytes, which can be confused with bronchioloalveolar carcinoma and bronchogenic adenocarcinoma, tend to be few, to be in tightly cohesive sheets and to have relatively normal nuclear/cytoplasmic (N:C) ratios. Nucleoli may be prominent. The possibility that atypical cells resembling adenocarcinoma are reactive type 2 pneumocytes should be carefully considered in cases in which there is clinical evidence of lung trauma or infection (e.g., intensive care unit lung).
Squamous metaplasia of the lower respiratory tract is common following lung irritation. Squamous metaplasia developing in association with diffuse alveolar damage (DAD) can be extensive and may resemble squamous cell carcinoma.2 Arguably, the metaplastic process is similar to that which occurs in the transformation zone of the cervix uteri, that is, basal (reserve) cell hyperplasia followed by immature and mature squamous metaplasia. Basal cell hyperplasia is characterized histologically by an increase in the basal cell layers from one to three or more with retention of the overlying columnar cells. Basal cells do not normally exfoliate but can be found in association with chronic bronchial irritation, infections, following tracheobronchial instrumentation, and, occasionally, with no known antecedent cause. In cytologic material basal cells tend to occur as tightly cohesive clusters of uniform small cells with scant cytoplasm and mildly hyperchromatic nuclei. They can be mistaken for cells shed by small cell carcinoma. Small cell carcinoma cells, however, are not cohesive and have hyperchromatic, molded, irregular nuclei with characteristic chromatin.
Immature metaplastic squamous cells occur as small sheets of uniform cells with evenly distributed uniform nuclei and a moderate amount of dense basophilic cytoplasm. They can be confused with the similar bronchiolar epithelial cell sheets, but metaplastic squamous epithelial cells are usually found in sputum, rather than in fine-needle aspirates, and have larger nuclei and more obvious cytoplasmic rims than bronchiolar epithelial cells. Mature metaplastic squamous cells are smaller and have denser and more eosinophilic cytoplasm than oropharyngeal squamous cells. Occasionally, small, single, highly keratinized cells with smudged, hyperchromatic nuclei that can be confused with metaplastic or dysplastic squamous cells are found in inadequate (i.e., no deep cough histiocytes) sputum specimens. These cells presumably represent degenerative changes in oropharyngeal squamous cells and are of little clinical significance.
Dysplastic immature metaplastic squamous cells display less intercellular cohesion, more cytoplasmic eosinophilia, and more nuclear variability than immature metaplastic squamous cells. Mildly dysplastic cells tend to be more cohesive and display less nuclear variability than severely dysplastic cells. Severely dysplastic squamous cells are poorly cohesive, have dense, irregular, eosinophilic cytoplasm, and pleomorphic hyperchromatic nuclei. They are difficult to distinguish from malignant squamous cells, which they may accompany. In general, dysplastic squamous cells tend to display greater cell cohesion and lesser cytoplasmic and nuclear pleomorphism than malignant squamous cells.
Preparation Techniques
Sputum
Sputum is a complicated mixture of mucus, debris, inflammatory cells, and exfoliated epithelial cells from upper and lower respiratory tracts. Sputum is expectorated spontaneously by patients with respiratory tract infections, by many cigarette smokers, and by patients with various diseases such as chronic bronchitis and bronchiectasis. An adequate sputum specimen, for the purpose of cytologic examination, must contain alveolar (deep cough) macrophages or dust cells. Nonsmokers without respiratory disease rarely produce an adequate specimen; sputum produced by these patients is usually saliva. Adequate sputum specimens can be induced by inhalation of heated hypertonic saline aerosol mist.3,4 The minimum requirement for acceptable sensitivity in the detection of malignancy is three adequate single sputum specimens.5 Sputum specimens can be processed fresh but are usually sent to the cytology laboratory in an alcohol-based fixative such as 50% ethanol, 70% ethanol, or 50% ethanol with 2% carbowax (Saccomanno fixative).6,7 Common methods of sputum preparation include the pick-and-smear method, sputum homogenization and cell concentration (Saccomanno technique),6,7 cell-block preparation (paraffin embedding following addition of a coagulative fixative such as Bouin’s fixative, or formalin fixation followed by addition of glycerin jelly or agar, or formalin fixation after addition of plasma)8,9 and monolayer techniques.10,11 The choice of sputum preparation method depends on the laboratory. No method is indisputably superior.12,13 The combination of two methods, such as direct smear of bloody particles in the sputum sample followed by cell-block preparation, may be the best compromise, at least in busy laboratories with few resources.
Bronchial Brush Cytologic Material
The flexible fiberoptic bronchoscope allows for most centrally and many peripherally located neoplasms to be directly observed and brushed. Bronchial brushing should be performed before bronchial biopsy to avoid contamination of the specimen with blood. The specimen obtained by direct bronchial brushing consists of mucus, respiratory mucosal epithelial cells, macrophages, and, if a lesion is sampled, tumor cells. In the classic method of bronchial brush preparation, the mucus-rich material adherent to a bronchial brush is smeared by rolling the brush over the central two thirds of a clear glass slide. The slide is immediately immersed in 95% ethanol. A delay in ethanol fixation results in air-drying artifact (nuclear enlargement, hypochromasia, and nuclear and cytoplasmic degeneration) and an uninterpretable specimen. Monolayer techniques can replace or be used with the direct smear.10,11 The choice of preparation technique for any cytologic material depends on laboratory resources. The direct smear (classic method) is the least expensive and, arguably, as good as the more expensive monolayer techniques.
Bronchial Lavage
Following bronchial brushing and usually before biopsy, or if no intrabronchial lesion can be seen, a bronchus can be washed with 5 to 10 mL of sterile isotonic saline. The fluid, aspirated back through the bronchoscope, is best preserved in a Saccomanno type fixative. It can be prepared by cytocentrifugation, membrane filtration, cell block preparation, and monolayer techniques. It contains mucus, bronchial epithelial cells, macrophages and other inflammatory cells, and, if a neoplasm is present, tumor cells. Not infrequently bronchial wash material contains microbiopsies, best studied in cell block preparations.
Bronchial Submucosal Fine-Needle Aspiration
Fine-needle aspiration biopsy can be performed through a flexible fiberoptic bronchoscope.14 Lesions beneath intact bronchial mucosa and enlarged hilar/subcarinal lymph nodes are amenable to this technique.15 Enlarged hilar/mediastinal lymph nodes can be aspirated through esophageal mucosa under endoscopic ultrasound (EUS) control.16–19 Preparation of the aspirated material is similar to that of fine-needle aspiration biopsy.
Bronchoalveolar Lavage
A flexible fiberoptic bronchoscope is wedged in an appropriate bronchus, usually the lingula or right middle lobe, and the parenchyma supplied by that bronchus washed in aliquots of 50 to 75 mL of sterile isotonic saline to a total of 200 to 300 mL.20 About half this fluid, largely from alveoli, is recovered through the bronchoscope. It contains alveolar macrophages, a few lymphocytes, and occasional neutrophils. Bronchial epithelial cells may be present.21 A cell count can be performed manually or by flow cytometry or the relative proportion of each cell type can be estimated by counting 100 to 200 cells on a stained slide. The material is prepared by cytocentrifugation, membrane filtration, cell block preparation, or monolayer techniques. BAL is of particular utility in the diagnosis of opportunistic pulmonary infections such as pneumocystis and cytomegalovirus pneumonia22 and in the investigation of interstitial lung disease. It is arguably underutilized in the investigation of lung malignancies.23,24
Transthoracic Fine-Needle Aspiration
Needle aspiration for diagnosis of lung lesions was first done in the United States in the 1920s.25 The technique did not win favor partly because tissue biopsy was preferred and partly because of the fear of needle track implantation. European physicians, particularly at the Karolinska Institute in Sweden,26 pioneered the use of fine (22-gauge) needles for the aspiration of lung lesions. Lung fine-needle aspiration biopsy is best performed under diagnostic imaging control by a radiologist or pulmonary physician.27 Because of the definite risk of pneumothorax and the lesser risk of significant pulmonary hemorrhage, aspiration should only be done where a thoracic surgeon is available. Local anesthesia of the pleura is desirable. For most lesions a 22-gauge, 90 mm disposable lumbar puncture style needle is suitable.28 The needle trocar prevents contamination of the specimen by tissues through which the needle passed. The aspirated material can be smeared directly on clear glass slides with air-drying or instant fixation in alcohol,29 or it can be placed in an alcohol fixative for later preparation. Hazards of transthoracic fine-needle aspiration include pneumothorax, death, hemorrhage, and implantation of the tumor.30 Pneumothorax is common and usually small.31 Death is very rare and probably due to air embolism. Significant pulmonary hemorrhage is uncommon. A few cases of implantation along the needle track (implantation metastasis) have been reported.32–36 Implantation metastases are associated with use of large-bore needles and core biopsy devices, with numerous passes of the needle into the carcinoma and with the absence of normal parenchyma overlying the lesion.37 Prudent precautions to minimize the risk of implantation metastasis include the use of fine needles (22 gauge or smaller), avoidance of multiple passes into the tumor, and avoidance of aspiration of a tumor that cannot be reached by passage of the needle through normal parenchyma.38 Transthoracic fine-needle aspiration biopsy is a remarkably useful and accurate technique for the diagnosis of lung carcinoma27 but is of less use in the exclusion of malignancy.28,39
Endoscopic Ultrasound-Guided Fine-Needle Aspiration Biopsy16–18,40
Endoscopic ultrasound-guided fine-needle aspiration biopsy of mediastinal lymphadenopathy is proving to be a sensitive and specific modality41 in the staging of non–small-cell lung carcinoma. It is superior to endoscopic ultrasound alone and to computed tomography alone. To date, complications of the procedure have been few.
Squamous Cell Carcinoma
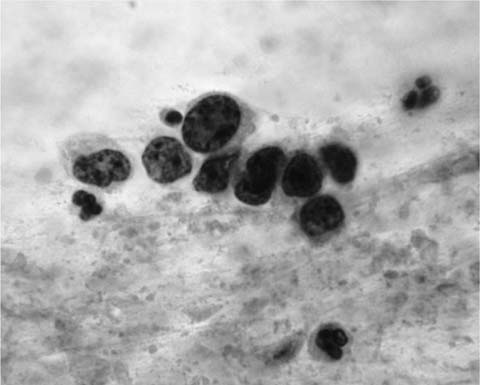
“Squamous cell carcinoma (epidermoid carcinoma) of the lung is a malignant epithelial tumor with the differentiating features of squamous epithelium: keratinization, intercellular bridges, or both.”42 In sputum smears well-differentiated (keratinizing) squamous cell carcinoma presents as single, isolated cells with bizarre cytoplasmic shapes, intense cytoplasmic eosinophilia (keratinization), and deeply hyperchromatic, angular nuclei in a background of necrotic and keratinous debris (Fig. 27–4). Intercellular bridges can occasionally be identified in small cell groups found in a smear and more commonly in microbiopsies found in cell block preparations. In bronchial brush, bronchial wash and lung fine-needle aspirates, keratinized malignant cells tend to be accompanied by less well-differentiated, nonkeratinized cells with rounder, less irregular and less hyperchromatic nuclei, more obvious nucleoli and dense basophilic or amphophilic cytoplasm with sharply defined cytoplasmic borders (Fig. 27–5).
FIGURE 27–4 Keratinizing squamous cell carcinoma (sputum, Pap stain).
The cells of nonkeratinizing squamous cell carcinoma occur both singly and in irregular, disorderly three-dimensional clusters. They have well demarcated cytoplasmic borders, eosinophilic to basophilic cytoplasmic staining, and, occasionally, a difference in density between outer and inner cytoplasmic layers (ecto- and endocytoplasmic differentiation). Their hyperchromatic, irregular, round to oval nuclei may have prominent nucleoli (Fig. 27–6). The cells of nonkeratinizing squamous cell carcinoma can be confused with those shed by other lung non–small-cell carcinomas. Confident diagnosis of squamous cell differentiation usually requires identification of at least a few cells with unequivocal cytoplasmic keratinization. Distinguishing squamous cell carcinoma from other lung non–small-cell carcinomas is often easier in sputum than in other cytologic material. Cells with angular, hyperchromatic nuclei and pronounced cytoplasmic eosinophilia, characteristic of squamous cell carcinoma in sputum, can usually be differentiated from three-dimensional clusters of cells with prominent nucleoli and delicate cytoplasm characteristic of adenocarcinoma. Small celled squamous cell carcinoma can be mistaken for small cell neuroendocrine carcinoma. Separation of these entities may require immunohistochemical stains. Squamous cell carcinoma does not express neuroendocrine markers or thyroid transcription factor-1 (TTF-1)43 and does express high molecular weight keratin; small cell neuroendocrine carcinomas often express neuroendocrine markers and TTF-1 and are negative for high molecular weight keratin. The cytologic features of basaloid-squamous cell carcinoma are described later in the chapter (see Large Cell Carcinoma). Although unusual in the general population44,45 well-differentiated squamous cell carcinoma of the lung in Okinawa is frequently infected with human papilloma virus.46 Malignant mesenchymal tissue such as cartilage and bone may occur in association with squamous cell carcinoma and, less frequently, adenocarcinoma and adenosquamous carcinoma (carcinosarcoma).47 The malignant mesenchymal tissue is infrequently recognized in cytologic, material resulting in a cytologic diagnosis of pleomorphic carcinoma, not otherwise specified.
FIGURE 27–5 Squamous cell carcinoma (bronchial brush, Pap stain).
FIGURE 27–6 Nonkeratinizing squamous cell carcinoma (bronchial brush, Pap stain).
Adenocarcinoma
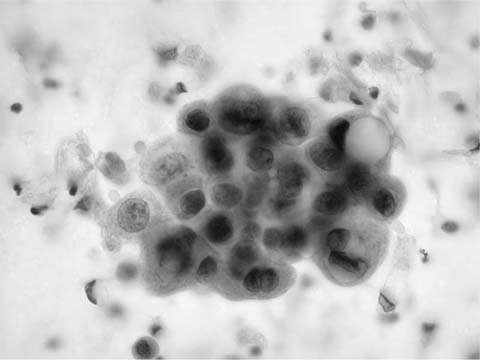
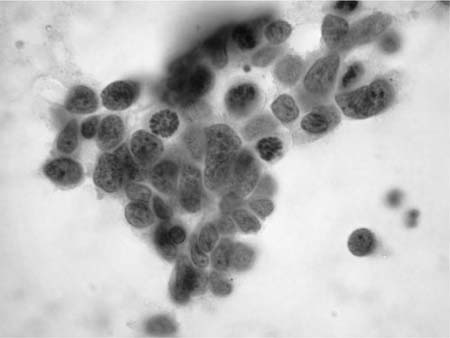
“Adenocarcinoma of the lung is a glandular epithelial malignancy manifesting tubal, papillary, or acinar growth patterns or a solid growth pattern with mucin production.”48 Adenocarcinoma, particularly in sputum, classically presents as cohesive, three-dimensional clusters of relatively uniform cells with delicate, finely vacuolated cytoplasm, inconspicuous cytoplasmic margins and vesicular, rounded nuclei with prominent nucleoli (Fig. 27–7).49 Irregular, round nuclear contours (cerebriform or “voluptuous” nuclei) are characteristic of adenocarcinoma and differ from the angularity of squamous cell carcinoma nuclei, a feature most often appreciated in sputum. Prominent nucleoli are usually present in adenocarcinoma, are often present in otherwise not classified non–small-cell carcinoma, are rare, at least in sputum, in squamous cell carcinoma and are not observed in small cell carcinoma. Three-dimensional adenocarcinoma cell clusters (Fig. 27–8) are usually accompanied by single cells with similar cytoplasmic and nuclear features. Granular necrotic debris (tumor diathesis) may be present in the background. Poorly differentiated adenocarcinomas have less well-defined cytoplasm, more variable nuclei, coarsely granular chromatin and macronucleoli. Acinar and papillary adenocarcinoma are cytologically similar.50 The cytologic features of poorly differentiated adenocarcinoma, poorly differentiated squamous cell carcinoma, and large cell carcinoma overlap making it difficult to distinguish them in routinely stained cytologic material. Thus these tumors are frequently classified under the rubric “non–small-cell carcinoma.” Identification of adenocarcinoma cells in sputum tends to be associated with a poor prognosis, possibly because the cells do not appear in sputum until metastasis to hilar or mediastinal lymph nodes has occurred.51 A false-positive cytologic diagnosis of lung adenocarcinoma can occur in association with pulmonary infection, diffuse alveolar damage, and pulmonary infarct,52 particularly if the diagnosis is based on sputum cytology in the absence of clinical information.
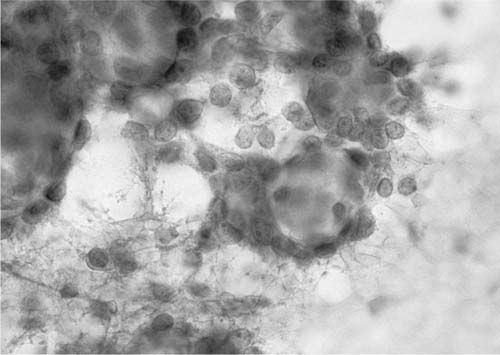
Small-sized (“early,” less than 2 cm diameter) adenocarcinomas of the lung demonstrate larger and more variable nuclei than those of localized bronchoalveolar adenocarcinoma53 (see later). Bronchioloalveolar carcinomas (BACs) (Fig. 27–9) are adenocarcinomas “in which cylindrical tumor cells grow upon the walls of preexisting alveoli…. The key feature is preservation of the underlying architecture of the lung.”48 BAC is defined as “an adenocarcinoma with a pure bronchioloalveolar growth pattern and no evidence of stromal, vascular or pleural invasion.” BAC is subdivided into nonmucinous (“A nonmucinous adenocarcinoma with Clara and/or type II pneumocytes growing along alveolar walls and without stromal invasion”), mucinous (“A mucinous adenocarcinoma composed of tall columnar cells, with varying amounts of cytoplasmic mucin, which typically displace the nucleus to the base of the cell, growing along alveolar walls and without stromal invasion”), and mixed mucinous and nonmucinous or indeterminate types [“An adenocarcinoma with a mixture of mucinous and nonmucinous cells or one in which it is impossible to separate the two cell types. The tumor grows along alveolar walls without stromal invasion”).54 In an alternate classification, Goto et al55 subdivides BAC into type I (“bronchioloalveolar adenocarcinoma without fibrotic foci”), type II (“sclerosing bronchioloalveolar adenocarcinoma without elastic framework destruction”), and type III (“early invasive adenocarcinoma”). The histologic diagnosis of BAC depends on the triad of tissue pattern (neoplastic cell growth along intact alveolar septa), absence of stromal invasion, and bland mucinous or nonmucinous neoplastic cells. Cytologic diagnosis depends largely on cell features, although growth along the intact alveolar septa can sometimes be identified in microbiopsies found in cell blocks, but is often difficult.
FIGURE 27–7 Adenocarcinoma (sputum, Pap stain).
FIGURE 27–8 Adenocarcinoma (bronchial brush, Pap stain).
The cytologic distinction of BAC from adenocarcinoma with BAC features or from atypical adenomatous hyperplasia56 may be impossible. BAC cells are bland, tend to occur in three-dimensional clusters, and are difficult to differentiate from normal and reactive bronchiolar epithelial cells.57,58 The cells of mucinous (secretory) bronchioloalveolar carcinomas have abundant, vacuolated cytoplasm, bland, folded nuclei, and inconspicuous nucleoli.59 The presence of nuclear grooves and prominent extracellular mucus may distinguish mucinous from nonmucinous BAC.60 Tumor cell clusters are associated with histiocytes and occasional more obviously neoplastic single cells or cell clusters. Round intranuclear cytoplasmic inclusions may be present in a few of the tumor cell nuclei. The cells of nonmucinous (nonsecretory) BAC have scanty cytoplasm and occur in monolayered sheets but otherwise resemble the mucinous form. The cytologic patterns are similar in exfoliated material and fine-needle aspirates, although monolayered cell sheets are larger and more numerous in the latter. Nonmucinous, but not mucinous, BAC is marked with TTF-1 and cytokeratin (CK)20.61 The cytologic diagnosis of BAC often depends on a high index of suspicion. A clinical history of radiologic nonresolving pneumonia, particularly in an afebrile host, should raise the differential diagnostic possibility of mucinous BAC. Three-dimensional cell clusters accompanied by foamy histiocytes and mucus and associated with occasional more pleomorphic neoplastic cells, together with close scrutiny of unequivocal normal tracheobronchial epithelial cells compared with putative neoplastic cells, may facilitate diagnosis. It may be impossible to confidently distinguish BAC from reactive bronchiolar epithelium62 or from adenocarcinoma.63,64
Bronchioloalveolar carcinoma may occur in children, particularly in association with congenital cystic adenomatoid malformation.65
The differential diagnosis of BAC includes, along with reactive bronchiolar epithelium and well-differentiated adenocarcinoma,66 atypical adenomatous hyperplasia,56 mucoepidermoid carcinoma, sclerosing hemangioma, alveolar adenoma,67 mucinous cystadenocarcinoma, adenocarcinoma with BAC features, and metastatic enteric (mucinous) adenocarcinoma.
FIGURE 27–9 Bronchioloalveolar carcinoma (fine-needle aspirate, Pap stain).
Frequently the differential diagnosis of a lung neoplasm includes both primary and metastatic adenocarcinoma. The distinction between a primary lung cancer and metastatic disease may require the use of immunocytochemical stains. An immunocytochemical panel including TTF-1, PE 10 (an antibody against human surfactant proteins), and CK7 and CK20, among others, may be used in the attempt to distinguish primary from secondary adenocarcinoma.68,69
Mucinous cystadenocarcinoma of the lung (Fig. 27–10) is an uncommon neoplasm similar to mucinous cystadenocarcinoma of the pancreas, appendix, and ovary. It is composed of a cyst-like mass of extracellular mucus associated with a relatively sparse, malignant epithelial lining (in contrast, BAC is not cystic). The presence of copious extracellular mucin in a lung fine-needle aspirate, particularly if the lesion has a cystic appearance in imaging studies, should raise the possibility of a mucinous cystadenocarcinoma even if there is little malignant epithelium in the aspirate.70,71
Endodermal tumor (well-differentiated fetal adenocarcinoma) of the lung was first described by Kradin et al72 in 1982 as a well-circumscribed malignant neoplasm composed of branching tubules lined with a single layer of pseudostratified columnar epithelium with clear or granular cytoplasm. The tumor has been divided into high grade and low grade.73 Fine-needle aspirate features of low-grade endodermal tumor include single cells and clusters of uniform epithelial cells with scant granular to clear cytoplasm, a dual cell population of smaller oval to columnar cells with hyperchromatic nuclei and larger round to oval cells with slightly open nuclei and small nucleoli, and gland differentiation, which is more obvious in the cell block.63,74,75 High-grade endodermal tumor resembles biphasic pulmonary blastoma (see below).
Small Cell Carcinoma
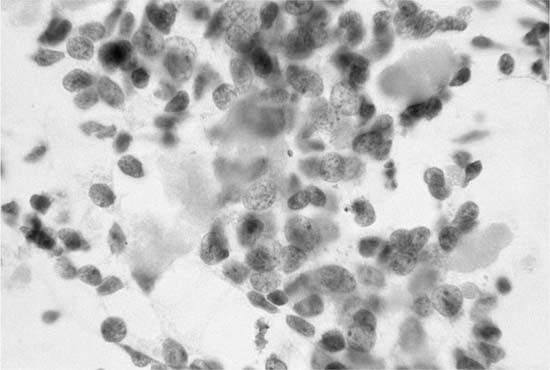
“Small cell carcinoma of the lung is a high-grade malignant epithelial tumor with characteristic cytologic features of scant cytoplasm, finely granular chromatin, absent or inconspicuous nucleoli, and frequent mitoses.”76 The International Association for the Study of Lung Cancer subclassified small cell carcinoma into three categories: small cell carcinoma, mixed small cell/large cell carcinoma, and combined small cell carcinoma (small cell carcinoma with admixed components of squamous cell and/or adenocarcinoma). The latter two categories are uncommon: mixed small cell/large cell carcinoma, 4 to 5%, and combined small cell carcinoma, 1 to 3% of small cell carcinomas.76 Small cell carcinoma, in cytologic material, typically presents as loose aggregates of small (two to three times the size of a lymphocyte) cells with hyperchromatic, pleomorphic nuclei, scant cytoplasm and no nucleoli commonly accompanied by necrosis and crush artifact.77,78 The malignant nuclei tend to mold around one another. Mitotic figures may be observed, particular in large cell aggregates. Malignant cell clusters with nuclear molding are particularly prominent in sputum (Fig. 27–11). Necrosis and apoptotic debris, nuclear crush artifact, and mitotic activity are more commonly found in bronchial brush cytologic material (Fig. 27–12). Small, blue, paranuclear inclusions, possibly representing phagocytosed apoptotic bodies, have been described in 1 to 4% of malignant cells in air-dried Romanovsky-stained fine-needle aspirate smears obtained from small cell carcinoma.79 Histologic and cytologic features of small cell carcinoma are similar.80 Small cell carcinomas typically arise in large bronchi and, at the time of diagnosis, have metastasized to hilar/mediastinal lymph nodes. Cytologic diagnosis is usually based on sputum and/or bronchial brush/wash cytology. Fine-needle aspiration biopsy diagnosis is less common.81 The diagnosis of small cell carcinoma based on fine-needle aspiration of a peripheral lung lesion should be made with caution. Peripheral spindle cell carcinoids share some of the cytologic features of small cell carcinoma. Unlike small cell carcinoma, typical carcinoids lack necrosis, mitotic activity, nuclear pleomorphism, and, usually, crush artifact.82 A peripheral lung lesion composed of small, elongated cells with scant cytoplasm and hyperchromatic nuclei is more likely a spindle cell carcinoid than small cell carcinoma. A centrally placed lesion composed of small cells without conspicuous nuclear pleomorphism, necrosis, and mitotic activity is more likely to be a central carcinoid than small cell carcinoma.
FIGURE 27–10 Mucinous cystadenocarcinoma (fine-needle aspirate, Pap stain).
Along with typical and atypical carcinoid, the differential diagnosis of lung small cell carcinoma includes basal (reserve) cell hyperplasia, lymphocytes, lymphoma, small cell adenocarcinoma, basaloid squamous cell carcinoma, non–small-cell bronchogenic carcinoma, large cell neuroendocrine carcinoma,83 metastatic malignancies, and rare lesions such as neuroectodermal tumors and primary lung sarcomas. Basal cell hyperplasia occurs as compact clusters of small, uniform cells with scant to moderate cytoplasm and small nucleoli. Lymphocytes tend to occur singly rather than in loose aggregates, have a thin rim of cytoplasm, and are not accompanied by necrosis, nuclear crush artifact or mitotic figures. Cells exfoliated from lymphoma may exhibit most or all the cytologic features of small cell carcinoma, and without clinical information or immunocytochemical stains may be impossible to confidently differentiate from small cell carcinoma. Small cell adenocarcinoma and other non–small-cell carcinomas, tend to exhibit at least some intercellular adhesion, a moderate amount of cytoplasm, and, most importantly, nucleoli. Nuclear crush artifact and nuclear molding are inconspicuous or absent.
FIGURE 27–11 Small cell carcinoma (sputum, Pap stain).
Compact clusters of small tumor cells with obvious nucleoli are more likely non–small-cell carcinoma than small cell carcinoma. Occasionally it may be impossible to distinguish small cell carcinoma, particularly of the mixed small cell/large cell and combined varieties, from non–small-cell carcinoma despite careful review of both cytologic and histologic material. Diagnostic immunocytochemistry may be of some use in such cases. Neuron-specific enolase (NSE) and synaptophysin are present in most small cell carcinomas; chromogranin is present in ~50%. Most small cell carcinomas, unlike non–small cell lung carcinomas, are negative for both CK7 and CK20.84 Most small cell carcinomas, unlike lymphoma and metastatic small cell tumors, are positive for TTF-1.85 Patients with small cell carcinoma uncommonly present with pleural effusions. The cytomorphology of small cell carcinoma in effusions is similar to that seen elsewhere. Positive immunohistochemical stains for TTF-1 or chromogranin may be of particular value in confirming a tentative diagnosis of small cell carcinoma in pleural fluid.86 Under unusual circumstances and in a small minority of patients, a trial of small cell carcinoma chemotherapy may serve to differentiate small cell carcinoma, which responds to therapy, from nonresponsive, non–small-cell carcinoma.
Large Cell Carcinoma
Large cell carcinoma is defined as “an undifferentiated malignant epithelial tumor that lacks the cytologic features of small cell carcinoma and glandular or squamous differentiation. The cells typically have large nuclei, prominent nucleoli and a moderate amount of cytoplasm.”87 Histologic variants of large cell carcinoma include large cell neuroendocrine carcinoma, combined large cell neuroendocrine carcinoma, basaloidsquamous carcinoma, lymphoepithelioma-like carcinoma,88 clear cell carcinoma, giant cell carcinoma, and large cell carcinoma with rhabdoid phenotype.89,90 Cytologic examples of classic large cell carcinoma consist of moderate to large, poorly cohesive, typically polygonal cells with large vesicular nuclei, a high nuclear/cytoplasmic ratio, and, usually, prominent nucleoli (Fig. 27–13). Features of keratinization, such as marked eosinophilia and dense cytoplasmic margins, and of glandular differentiation, such as mucus vacuoles, are absent. Mitotic figures may be identified and there is frequently a background of necrotic debris. Many cases of large cell carcinoma are poorly differentiated adenocarcinomas and squamous cell carcinomas in which cytoplasmic differentiation is too indefinite to permit diagnosis.91,92 Should the cytologic material display an “organoid” pattern, the differential diagnosis of large cell neuroendocrine carcinoma (see later) should be considered.
FIGURE 27–12 Small cell carcinoma metastatic to liver (fine-needle aspirate, Pap stain).
Large cell neuroendocrine carcinoma is defined as “a large cell carcinoma showing histologic features such as organoid nesting, trabecular, rosette-like and palisading patterns that suggest neuroendocrine differentiation and in which the latter can be confirmed by immunohistochemistry or electron microscopy.”87 Criteria for the cytologic diagnosis of large cell neuroendocrine carcinoma proposed by Wiatrowska et al93 and refined, particularly on fine-needle aspirates, by Yang et al83 include the above features of large cell carcinoma plus features of neuroendocrine carcinoma. The diagnosis of high-grade carcinoma is based on the presence of necrosis (tumor diathesis) and mitotic figures. The diagnosis of a large cell tumor is based on nuclear size (greater than 2.5 times the size of an erythrocyte) and prominent nucleoli. The diagnosis of neuroendocrine differentiation is based on an organoid pattern, fine (usually) chromatin, crush artifact, and positive neuroendocrine immunocytochemical stains such as chromogranin and synaptophysin.94 Large cell neuroendocrine carcinoma and large cell carcinoma not otherwise specified share features of large nuclear size, prominent nucleoli, and scant cytoplasm. Large cell neuroendocrine carcinomas and low-grade neuroendocrine carcinomas such as carcinoid and atypical carcinoid share the features of finely stippled chromatin, single cell pattern, bare nuclei, and a trabecular or rosette pattern plus positive neuroendocrine immunocytochemical stains. Crush artifact is more likely to occur in association with small cell carcinoma and large cell neuroendocrine carcinoma than with typical and atypical carcinoid. Neuroendocrine markers found in carcinoids can also be found in other lung tumors, notably large cell carcinoma not otherwise specified, and are not specific for high-grade neuroendocrine lung carcinomas.95 Thus the diagnosis of large cell neuroendocrine carcinoma requires more than positive neuroendocrine immunocytochemical stains. Diagnosis can be difficult, particularly in fine-needle aspirate material.83,96 Large cell neuroendocrine carcinoma can be confused, both by cytology and histopathology, with basaloid squamous carcinoma (see later). TTF-1 expression tends to occur in large cell neuroendocrine carcinoma but not in basaloid carcinoma, whereas cytokeratin 34 E12 expression tends to occur in basaloid but not large cell neuroendocrine carcinoma.97
FIGURE 27–13 Large cell carcinoma (bronchial brush, Pap stain).
Basaloid-squamous cell carcinoma of the bronchus is a variant of squamous cell carcinoma with both basaloid and squamous features.98,99 The lung is an uncommon site. The tumor typically presents as metastatic disease to a cervical lymph node from an upper aerodigestive tract primary. The squamous element of the tumor resembles squamous cell carcinoma seen elsewhere. The basaloid component is made up of lobular islands of small crowded cells without nucleoli and with scant cytoplasm surrounded by abundant basement membrane–like material somewhat resembling adenoid cystic carcinoma (Fig. 27–14). Fine-needle aspirate material contains a similar admixture of small basaloid cells, basement membrane–like material, and malignant squamous cells. The differential includes small cell carcinoma, adenoid cystic carcinoma, adenosquamous cell carcinoma, and undifferentiated non–small-cell carcinoma.100–102 Cytologic diagnosis is possible if the mixture of cellular elements is recognized.
Lymphoepithelioma-like carcinoma rarely occurs in the lung of Epstein-Barr virus positive Chinese patients.88,103,104 The carcinoma resembles the far more common nasopharyngeal carcinoma in both histologic and cytologic features. It is composed of undifferentiated malignant cells with large vesicular nuclei, prominent nucleoli, and scant cytoplasm in a syncytial pattern, usually with a background of lymphoid cells. Giant cell carcinoma is a large cell carcinoma containing highly pleomorphic, bizarre, multinucleate cells often with intracytoplasmic neutrophils87,105,106 (Fig. 27–15). It usually arises peripherally, is highly aggressive, and has a poor outcome. The cytologic appearance is similar to the histologic with dispersed pleomorphic large cells admixed with multinucleate giant cells, which may have intracytoplasmic neutrophils. The broad differential diagnosis includes metastatic germ cell carcinoma, adenocarcinoma,107 combined choriocarcinoma and adenocarcinoma,108 large cell lymphoma, melanoma, and malignant fibrous histiocytoma,109 among others.110
FIGURE 27–14 Basaloid squamous cell carcinoma (fine-needle aspirate, Pap stain).
Miscellaneous Pulmonary Neoplasms
Primary Salivary Gland-Type Tumors of the Lung111
Mucoepidermoid carcinoma is an uncommon neoplasm presumably arising from bronchial glands. High-grade mucoepidermoid carcinoma is difficult to distinguish from adenosquamous carcinoma.112 Adenosquamous carcinomas tend to occur in the lung periphery, and mucoepidermoid carcinomas occur centrally. Low-grade mucoepidermoid carcinomas are composed of mingled squamous, mucus-secreting, and intermediate cells.112–114 They resemble the more common salivary gland counterpart. Differential diagnosis includes reactive bronchial epithelial cells with associated squamous metaplasia, well-differentiated squamous carcinoma, and adenocarcinoma.
Bronchial adenoid cystic carcinoma resembles its salivary gland counterpart in cytologic, histologic, and clinical features. It is composed of small cells with hyperchromatic nuclei and scant cytoplasm. Unlike the loosely cohesive cells of small cell carcinoma, the cells of adenoid cystic carcinoma are found singly and in tight three-dimensional clusters. Essential for diagnosis is the presence of balls or cylinders of basement membrane material, which may be surrounded by tumor cells112,115 (Figs. 27–16 and 27–17). Differential diagnosis includes reserve cell hyperplasia, basaloid-squamous cell carcinoma, metastasis from a salivary gland primary, and small cell carcinoma.
Pulmonary Carcinoid
Typical pulmonary carcinoids are relatively common primary, submucosal, intrabronchial neoplasms. Carcinoid cells are rarely detected in sputum or bronchial brushes (unless the brush breaks through the mucosa). They can be identified by vigorous bronchial brushing or fine-needle aspiration. The cells of typical carcinoid are uniform, monomorphic, and single or in loose aggregates. They have delicate wispy cytoplasm, and uniform spherical nuclei with fine chromatin and inconspicuous nucleoli. Necrosis and mitotic activity are absent; if either is observed, the lesion is more likely an atypical carcinoid (see later). The bland, uniform cells of typical carcinoid are easily overlooked116,117 (Fig. 27–18).
FIGURE 27–15 Giant cell carcinoma (bronchial brush, Pap stain).
Peripherally located typical carcinoids tend to have elongated spindle or cigar-shaped nuclei, fine chromatin, and scant cytoplasm. They may be confused with small cell carcinoma, but small cell carcinoma does not commonly occur peripherally, the cells of small cell carcinoma are pleomorphic, and necrosis and mitotic figures may be present in small cell carcinoma but not in carcinoid.118 Similar cohesive, spindle-shaped cells have been identified in fine-needle aspiration material from a single pulmonary tumorlet.119
Atypical carcinoid occupies a niche between the relatively benign typical carcinoid and the highly malignant small cell carcinoma. Metastasis to hilar and mediastinal lymph nodes is common. The polygonal or fusiform cells of atypical carcinoid are larger than those of typical carcinoid, have hyperchromatic, mildly to moderately pleomorphic, occasionally molded nuclei, and may have prominent nucleoli. Important for diagnosis is the presence of necrosis or mitoses.120 Unlike small cell carcinoma, many (about two thirds) of primary lung carcinoids are negative for TTF-1.121
Oncocytic carcinoid tumor of the lung, a rare variant of carcinoid tumor, is composed of cells with regular nuclei and abundant, granular, eosinophilic cytoplasm.122 The differential includes other oncocytic tumors of the lung, both primary and metastatic,123 and granular cell tumor.124 A clear cell variant of pulmonary carcinoid has been reported.125
Sclerosing Hemangioma
Sclerosing hemangioma is a not uncommon peripherally located lung neoplasm with a marked female predisposition. It is composed of pale polygonal cells, surface epithelial cells, and transition forms between the two organized in papillary, fibrotic, vascular, and mixed patterns.126,127 Fine-needle aspirate features mimic those of nonsecretory BAC, including occasional intranuclear cytoplasmic inclusions. Bland mononuclear cells organized in clusters and papillae are mixed with fibrotic stromal tissue fragments. These features are best seen in cell block sections. Tumor cells are TTF-1, epithelial membrane antigen (EMA), and vimentin positive and cytokeratin negative.126,128,129 Differential diagnosis includes, as well as BAC130 and papillary adenocarcinoma,129 peripheral carcinoid. The bland mononuclear cells of sclerosing hemangioma lack the characteristic chromatin of carcinoid tumors.
FIGURE 27–16 Adenoid cystic carcinoma (fine-needle aspirate, Pap stain).
Pulmonary Hamartoma131,132
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree