Diagnostic Bronchoscopy, Transthoracic Needle Biopsy, and Related Procedures
INTRODUCTION
Gustav Killian reported his experience with the first bronchoscopy in 1898. Technological advances during the next century facilitated development of bronchoscopy as a pivotal diagnostic and therapeutic tool in pulmonary medicine. Although a number of bronchoesophagologists contributed to refinement of the technique based upon use of a rigid instrument, the advent of flexible fiberoptic bronchoscopy, pioneered by Ikeda in 1967, opened new horizons to clinicians. More recently, transthoracic needle biopsy (TTNB) has been added to the pulmonologist’s diagnostic armamentarium, although it is now most frequently performed by radiologists under CT guidance.
This chapter comprises an overview of bronchoscopy, TTNB, and related techniques. Following a general discussion of bronchoscopy and associated general instrumentation, indications for the technique and patient preparation are considered. Specific applications of diagnostic bronchoscopy are discussed. Subsequently, safety factors related to bronchoscopy and complications of the technique are reviewed. Finally, TTNB is described.
GENERAL INSTRUMENTATION
The initial bronchoscope, developed by Killian in Europe and further perfected by Chevalier Jackson in the United States, was a rigid metal tube that permitted either spontaneous or mechanical ventilation. With development of fiberoptic and advanced electronic technology, the flexible bronchoscope has, to a large extent, replaced the rigid bronchoscope for most diagnostic and some therapeutic indications. Therapeutic interventional bronchoscopy, including the use of rigid bronchoscopes, is discussed in Chapter 36.
 FLEXIBLE FIBEROPTIC AND VIDEOBRONCHOSCOPY
FLEXIBLE FIBEROPTIC AND VIDEOBRONCHOSCOPY
Although the optical resolution of early fiberoptic bronchoscopes was inferior to that of rigid devices, their flexibility, ease of manipulation, and simplicity of use, which permit rapid examination under topical anesthesia, have made flexible bronchoscopy the primary endoscopic procedure in pulmonary diseases.
Unlike the larger-bore rigid bronchoscope, the flexible bronchoscope varies from ultrathin – allowing for neonatal endoscopy – to larger, adult size therapeutic devices. The diameter of the working channel permits aspiration of secretions or introduction of accessories required for diagnostic purposes (see Bronchoscopy Technique). With flexible bronchoscopy, the patient’s ventilation is assured by airflow around the bronchoscope, between the external wall of the device and the tracheobronchial tree. Thus, the appropriate selection of bronchoscope size is crucial.
Fiberoptic systems have largely been replaced by videobronchoscopes, which utilize a miniaturized CCD camera at the tip of the scope that provides electronic transmission of images to a television monitor. Flexible bronchoscopes are more fragile and more prone to damage than are rigid metal instruments. Appropriate care and adherence to safety techniques during procedures, as well as during routine cleaning and maintenance of the instruments, help assure extended instrument life and reduce repair costs.
 ULTRATHIN BRONCHOSCOPES
ULTRATHIN BRONCHOSCOPES
Ultrathin bronchoscopes, flexible scopes with external diameters ≤3 mm, were initially developed for pediatric applications; however, these have now incorporated larger working channels, allowing for their use in the diagnosis of peripheral pulmonary lesions in adults.1,2 Ultrathin bronchoscopes can be advanced to more peripheral bronchi than conventional bronchoscopes under direct observation, allowing for examination of sixth- to eighth-generation bronchi. Ultrathin scopes may be particularly useful when combined with additional diagnostic tools, such as navigational bronchoscopy and radial ultrasound probes (see Ultraminiature Radial Probes and Navigational Bronchoscopy).
DIAGNOSTIC BRONCHOSCOPY ACCESSORIES
The working channel of the fiberoptic or videobronchoscope, although of relatively small diameter, allows the insertion of various diagnostic and therapeutic accessories.
 BIOPSY FORCEPS
BIOPSY FORCEPS
Simple visualization of lesions is usually not sufficient to determine a precise diagnosis and to guide management. Pathological confirmation through biopsy is frequently required. A variety of instruments with improved distal control (i.e., control beyond the tip of the bronchoscope) have been developed that permit tissue cutting and retrieval of biopsy specimens.
The cutting cups of biopsy forceps may be round or elliptic and may have smooth or jagged edges. The use of nonserrated edges, however, seems to reduce tissue trauma and the concomitant risk of bleeding. The biopsy procedure is simple and generally associated with only minimal complications in the case of a visible lesion. Even peripheral lesions, which are not visible through the bronchoscope, may be biopsied. With diffuse parenchymal or interstitial lung disease, specimens may be obtained without fluoroscopic guidance. With smaller or focal lesions, however, the diagnostic yield of biopsies increases when fluoroscopy is used. The development of new electromagnetic and remote guidance systems suggests that further improvement in the diagnostic yield of bronchoscopic biopsies can be expected.
 BRONCHIAL BRUSHES
BRONCHIAL BRUSHES
Lesions not accessible to direct biopsy with a forceps can at times be approached with a bronchial brush. This device consists of a rigid central wire surrounded by brushes of various sizes and shapes. To-and-fro movement of the brush against the adjacent tissue produces minor trauma but enables collection of ample specimens for cytological or microbiological analysis.
In some clinical circumstances, there is a need to obtain an uncontaminated specimen from the lower respiratory tract for microbiological studies. A brush protected by an additional sheath and tip may be passed through the working channel of the bronchoscope (protected brush specimen, as discussed later). In these cases, special attention is needed not to use an excessive amount of local anesthetic or saline lavage, since these solutions contain bacteriostatic material that may inhibit microbial growth. The diagnostic yield depends on use of proper technique, appropriate choice of brush, and careful collection and preservation of the specimen.
 NEEDLES FOR ASPIRATION AND BIOPSY
NEEDLES FOR ASPIRATION AND BIOPSY
The first performance of a transbronchoscopic needle aspiration (TBNA) through a rigid bronchoscope was reported by Schieppati in 1958. Wang et al.3 then developed a flexible needle technique using a fiberoptic bronchoscope in 1978. Initially, several models of needles were designed to obtain cytological material; subsequently, histological specimens from peribronchial mediastinal and hilar lymph nodes were obtained with larger-bore needles. These biopsy needles are also useful in the diagnosis of endobronchial and submucosal lesions and can serve as a complementary technique to percutaneous needle aspiration of peripheral pulmonary nodules or masses.
The tip of the needle is protected by a metal hub during the insertion and withdrawal to avoid damage to the flexible scope. Perforation of the working channel of the scope may occur if the needle is advanced in an exposed position. The diagnostic yield depends on two factors: Optimization of the bend of the tip of the bronchoscope and proper performance of bronchial wall puncture by the needle through the intercartilaginous space. Familiarity with the type of needle used increases the success rate.
TBNA is generally safe, although pneumothorax and hemomediastinum can occur. Clinically significant bleeding is extremely rare, particularly when a 22-gauge needle is used, even if a major vessel is inadvertently punctured or if the patient suffers from superior vena cava syndrome.
ENDOBRONCHIAL ULTRASOUND
Among the new diagnostic modalities available to chest physicians, endobronchial ultrasound (EBUS) has unquestionably had the most profound impact.4 Two major barriers to EBUS development existed: Ultrasound probe size and sound wave transmission in air-filled structures. Ultrasound engineering advances allowed the former barrier to be overcome. The latter was surmounted by developing an integrated, fluid-filled balloon surrounding the EBUS probe, thereby allowing for a sound wave–transducing medium interface to exist between the ultrasound probe and airway wall (i.e., ultrasonographic coupling).
Ultrasound frequency is an important consideration for EBUS application. Lower frequencies give better penetration depth with less resolution; higher frequencies provide better spatial resolution, but less penetration depth. For EBUS applications, the frequencies range from 7.5 to 30 MHz. Currently, there are three EBUS probes available for different applications: (1) Ultraminiature radial probes (20 and 30 MHz), (2) radial balloon probes (20 MHz), and (3) convex probe or curvilinear EBUS (CP-EBUS). Each of these is discussed in greater detail later.
 ULTRAMINIATURE RADIAL PROBES
ULTRAMINIATURE RADIAL PROBES
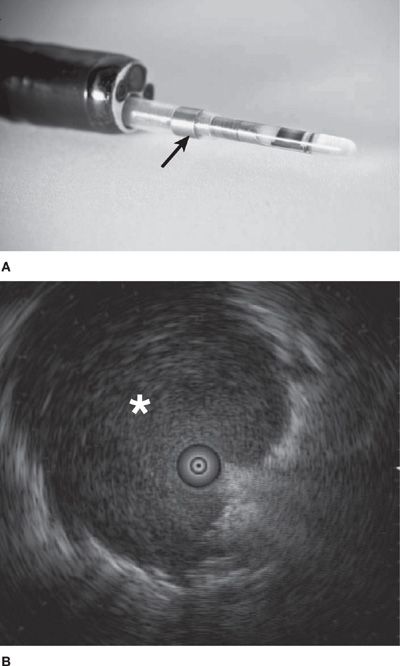
Ultraminiature EBUS (UM-EBUS) was developed to allow for improved assessment and sampling of peripheral pulmonary lesions (Fig. 35-1A). There are two ultraminiature radial probes currently available, with diameters of 1.4 and 2.0 mm, allowing insertion into bronchoscopes with working channels of 2.0 and 2.6 mm, respectively. When a lesion is reached with the probe, the usual normal lung “snowstorm” appearance is replaced by a focal ultrasound alteration that can be marked by fluoroscopy, a guide sheath (GS), or both fluoroscopy and GS (Fig. 35-1B and Video 35-1). After the lesion is localized, the GS can be left in place, allowing for guided biopsies of the lung using forceps, brush, or needle biopsy.
Figure 35-1 A. The ultraminiature (UM)-EBUS probe contains a circulating ultrasound crystal that provides a 360-degree view of the surrounding structures when full airway ultrasonographic coupling occurs. The probe is inserted through a guide sheath (arrow; Olympus Corporation, Tokyo, Japan), which can remain in the airway on UM-EBUS probe removal to allow for instrument guidance for biopsy. B. This UM-EBUS image demonstrates a focal lung lesion (asterisk) surrounding the probe.
Video 35-1 The ultraminiature (UM)-EBUS probe is used via the working channel of a fiberoptic bronchoscope to identify a focal lung lesion. When the lesion is reached with the probe, there is an alteration in the ultrasound image, with replacement of the usual normal lung “snowstorm” appearance by the presence of a denser focal lesion that surrounds the centrally located probe. Access at www.fishmansonline.com
 RADIAL BALLOON PROBE
RADIAL BALLOON PROBE
To overcome the ultrasonographic coupling problem with UM-EBUS probes in the central airways, a radial EBUS probe was developed; the probe is inserted into an outer sheath with a distal tip balloon. The balloon is filled with saline to provide a fluid medium to allow for sound wave transmission from the probe to the airway wall. The radial balloon EBUS (RB-EBUS; Olympus Corporation, Tokyo, Japan) probe provides <1-mm resolution with a 360-degree visualization of paratracheal and peribronchial structures (Fig. 35-1). Five to seven layers of the tracheal and proximal bronchial wall have been described using RB-EBUS.5
 CONVEX PROBE EBUS
CONVEX PROBE EBUS
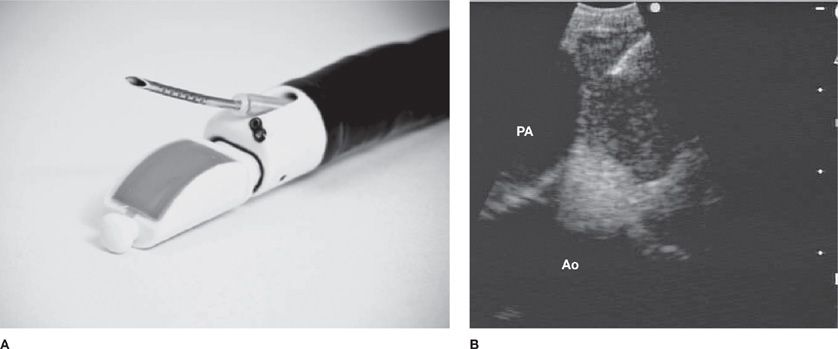
The convex probe EBUS (CP-EBUS) bronchoscope, introduced in 2005, has a built-in curvilinear ultrasound transducer with a larger distal diameter (6.9 mm) compared with a standard bronchoscope. White light videobronchoscopy occurs at a 35-degree oblique angle with EBUS at 90 degrees from the longitudinal axis. Dedicated biopsy needles (21 or 22 gauge) are inserted through the 2-mm working channel to perform aspirations of the target lesion (Fig. 35-2A). Real-time EBUS imaging displays needle penetration through the tracheobronchial wall into the target during the biopsy maneuver (Fig. 35-2B). If there is difficulty in achieving adequate EBUS images because of poor ultrasonographic coupling, a saline-filled balloon surrounding the transducer can be used to improve image quality. In addition, Doppler capabilities allow vascular structure differentiation, which minimizes the risk of unintended vascular puncture.
Figure 35-2 A. The convex probe (CP)-EBUS TBNA (BF-UC160 F-OL8; Olympus Corporation, Tokyo, Japan) videobronchoscope has an integrated ultrasound probe that scans 90 degrees perpendicular from the longitudinal axis, a 35-degree forward oblique video view, and a 2.0-mm working channel through which a dedicated biopsy needle can be passed. Distal tip dimples on the needle provide an echogenic surface to reflect ultrasound waves to allow needle visualization. B. This CP-EBUS image demonstrates a left paratracheal lymph node with the ascending aorta (Ao), the pulmonary artery (PA), and the needle present in the lymph node.
NAVIGATIONAL BRONCHOSCOPY
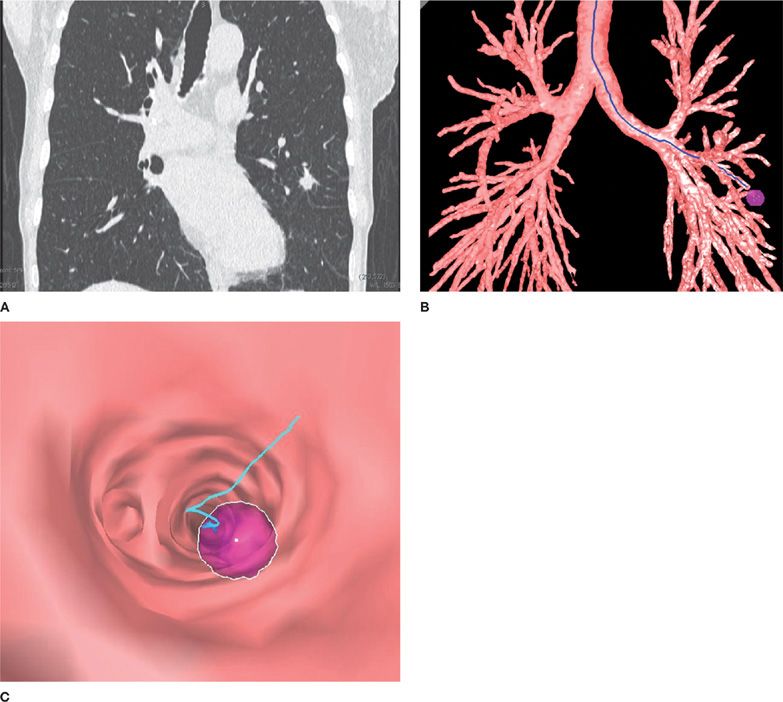
A recent advance in the evaluation of peripheral pulmonary lesions and mediastinal and hilar adenopathy has been the development of navigational approaches, such as electromagnetic navigational bronchoscopy (EMB) and virtual bronchoscopy (VB) (Fig. 35-3).6–9
Figure 35-3 Use of a virtual bronchoscopy–based system for navigation. A. Chest CT image of a spiculated left upper lobe pulmonary nodule. B. Three-dimensional reconstruction of the tracheobronchial tree with the nodule identified. C. Virtual bronchoscopic image demonstrating the pathway to the peripheral nodule.
EMB utilizes an electromagnetic board to generate a magnetic field around the patient, a magnetic sensor probe, an extended working channel, and three-dimensional integration of CT scan reconstruction and bronchoscopy position. In essence, this system works on the same triangulation principle as a global positioning system and allows the bronchoscopist to direct the FB through the airways to the target.
VB-based approaches utilize virtual navigation by creating a CT scan–based “road map” that can be overlaid onto real-time endoscopic images. Navigational systems can be used in conjunction with ultrathin bronchoscopies combined with radial EBUS probes and GSs to confirm that a lesion has been reached and to maintain the position for acquisition of diagnostic material. The role of navigational bronchoscopy in the evaluation of peripheral pulmonary nodules is discussed later.
In addition to diagnostic indications, navigational bronchoscopy is increasingly used for targeted cancer therapeutic delivery, including guided stereotactic radiosurgery, fiducial placement, or implantation of radiotherapy monitoring devices.10,11
Navigational bronchoscopy systems may be limited in general application by their high capital cost and training necessary for optimal system utilization. At the current time, the greatest experience and yield with these technologies has occurred in centers of excellence, with results unlikely to be reproducible in less experienced centers.
PATIENT PREPARATION AND MONITORING DURING BRONCHOSCOPY
Success of bronchoscopy, whether diagnostic or therapeutic, depends, in large part, on proper preparation of the patient, including relief of anxiety, muscle relaxation, cough suppression, and adequate anesthesia. Time spent in achieving these goals will be well worth it in reducing the risks of complications and in increasing the ease of performance of the procedure. As with any other procedure, analysis of the risk–benefit ratio helps reduce the complication rate. During and shortly after the procedure, appropriate monitoring of hemodynamic parameters (heart rate, rhythm, and blood pressure), oxygenation, and ventilation contributes to the safety of bronchoscopy.
Most flexible bronchoscopies are performed after patient premedication with sedative agents and the use of bronchoscopically instilled lidocaine for local anesthesia of the upper airway, larynx, and tracheobronchial tree. Most frequently, moderate sedation is achieved using a combination of a short-acting benzodiazepine (e.g., midazolam) and a narcotic agent (e.g., fentanyl). Intravenous propofol may also be used to provide moderate sedation and appears to provide similar results in terms of patient satisfaction and degree of hypoxia, with the advantage of a faster recovery time.12 Because the use of propofol can lead to deep sedation, it is important that these patients receive careful monitoring. Deep sedation (i.e., a deeper state of depressed consciousness with potential for compromised airway function and spontaneous respiration) and general anesthesia are increasingly being employed given the shift of diagnostic and therapeutic bronchoscopy toward more complex and lengthier diagnostic procedures.
Anticholinergic medication (e.g., atropine or glycopyrrolate) has been advocated by some to reduce the risk of vasovagal reactions and to minimize airway secretions, thereby allowing for better examinations of the tracheobronchial tree. However, in a large randomized trial comparing these two drugs with placebo, glycopyrrolate, but not atropine, led to a reduction in airway secretions.13 There was no significant reduction in cough, patient discomfort, oxygen desaturation, or procedure time with either drug. Current recommendations discourage the use of these agents during bronchoscopy.12
BRONCHOSCOPY TECHNIQUE
Central components of the routine bronchoscopic technique are discussed later.
 ASSESSMENT OF AIRWAY ANATOMY AND FUNCTION
ASSESSMENT OF AIRWAY ANATOMY AND FUNCTION
Thorough bronchoscopic evaluation begins with examination of the upper airways. Special attention should be paid to the integrity of air passages and the function of the nasopharynx and larynx. The vocal cords should be examined for the presence of polyps and tumors and for evidence of cord paralysis.
Once upper airway inspection is completed, a systematic evaluation of the lower respiratory tract should be performed. Critically important is the distinction among normal anatomy, anatomic variations without clinical significance, and frankly pathological conditions. These considerations have important implications regarding potential diagnostic and therapeutic approaches. For example, finding an abnormal branching of a bronchus may be of no clinical significance. On the other hand, such an abnormality could explain symptoms of frequent infections due to impaired ventilation and drainage of the affected area. Special skills and observational experience are required for bronchoscopic examination after surgery, especially following creative bronchoplastic procedures or lung transplantation.
Assessment of airway integrity, with special attention to dynamic changes in airway caliber during either relaxed breathing or forced expiration and coughing, may be crucial in determining appropriate therapeutic maneuvers. Flexible bronchoscopy is superior to rigid bronchoscopy for this assessment. Relaxation and prolapse of the membranous portion of the trachea and main bronchi secondary to destruction of elastic connective tissue may account for exacerbations of expiratory airflow obstruction. On the other hand, finding localized, posttraumatic chondromalacia has very different therapeutic implications. On the basis of these bronchoscopic determinations, the choice of performing an open surgical approach or bronchoscopic therapeutic correction may be made.
Bronchoscopic examination generally permits evaluation and localization of congenital or postsurgical pathological changes in bronchial integrity, such as tracheoesophageal or bronchopleural fistulas. Bronchoscopic observation and early diagnosis of bronchial rupture after chest trauma also greatly influence further therapy and prognosis. The same is true for evaluation of postsurgical anastomoses following reconstructive surgery or lung transplantation.
Advances in airway management of critically ill patients who require prolonged intubation or tracheotomy have resulted in a lower incidence of tracheal injuries. Tracheal injuries documented by bronchoscopy are not rare, however. Important complications of tracheotomy include tracheal stenosis, tracheomalacia, and tracheoinnominate artery fistula. Complications specific to the use of percutaneous tracheotomy, which is increasingly used in the intensive care unit, include flaps of cartilage protruding into the tracheal lumen and extraluminal placement of the tracheostomy tube. Such complications can have significant bearing on clinical outcome.
 EVALUATION OF TRACHEOBRONCHIAL MUCOSA
EVALUATION OF TRACHEOBRONCHIAL MUCOSA
Careful examination of the mucosal surface is crucial in the formulation of differential diagnosis. Rapid development of granulation tissue is frequently associated with reaction to a foreign body. Inflammatory mucosal reactions, although not very characteristic, should raise the possibility of mycobacterial infection, nonspecific viral and nonviral infections, and other granulomatous diseases, such as sarcoidosis.
The distinction between normal, pale-pink mucosa and hypervascular areas in the tracheobronchial tree may provide important diagnostic clues. Most frequently, changes in mucosal coloration are associated with an inflammatory reaction due to bronchitis. These findings are, however, very distinctive from small hemangiomas or vascular distentions due to compression by enlarged, neoplastic lymph nodes. Similarly, a network of small mucosal lymphatics may be visible, with lymphatic interruption due to surgery, radiation therapy, fibrosis, or malignancy. This is most frequently associated with local edema, which contributes to airflow obstruction. In addition, distinct and characteristic mucosal discoloration can be observed in Kaposi sarcoma.14–16
Ulcerations of the mucosa are more characteristic of Wegener granulomatosis or malignancy. Loss of the usual mucosal luster and presence of a roughened surface may alert the expert bronchoscopist to an early infiltrative or neoplastic process. Previously sustained injuries are characterized by the formation of mucosal and submucosal fibrosis, resulting in airway retraction or distortion.
Autofluorescence bronchoscopy (AFB) permits observation and analysis of tracheobronchial mucosal surfaces using the discriminant characteristic of tissue autofluorescence (Video 35-2). It is well known that when stimulated with light of a specific wavelength, normal tissues emit specific fluorescence. Changes in the structural integrity of the same tissues due to pathological processes modify or suppress the autofluorescence. The fluorescent emissions are too low in intensity to be seen by the human eye. With the use of a monochromatic light source, computer-controlled image analysis, and a sophisticated camera attached to a fiberoptic bronchoscope, the airways can be examined for varying degrees of autofluorescence as an indicator of early-stage malignant changes. The acquisition of images is obtained in real time and helps in the detection of minute areas of change in normal tracheobronchial mucosal fluorescence. Biopsies from areas of abnormal fluorescence increase the rate of detection of small, premalignant (dysplasia) or early malignant (carcinoma in situ) lesions in the tracheobronchial tree. Confirmation is provided by biopsy of the suspect or abnormal areas under direct bronchoscopic control, followed by pathological review.
Video 35-2 White light and autofluorescence bronchoscopy (AFB) demonstrating a focal airway lesion at the carina between the lingua and superior division of the left upper lobe. The lesion demonstrates abnormal autofluorescence, manifested by a brownish color on AFB. The remainder of the airway examination demonstrates normal green fluorescence. Access at www.fishmansonline.com
Although AFB may provide the ability to localize these early lesions with greater sensitivity than white light bronchoscopy (WLB),17–20 longitudinal studies demonstrate that only 0% to 9% of moderate dysplastic foci and 0% to 32% of severe dysplastic foci progress to CIS or invasive cancer,21–23 and 60% to 65% of moderate/severe dysplastic lesions regress or resolve spontaneously.21 The uncertainty of the natural history of central airway dysplastic lesions, combined with the increasing incidence of peripheral adenocarcinomas not accessible to bronchoscopic visualization, makes it unlikely that AFB will find a role as a routine screening tool for lung cancer in large populations.
Narrow band imaging (NBI) uses a unique filter to select light wavelengths that preferentially are absorbed by hemoglobin, thereby permitting superior microvasculature detection. Because angiogenesis occurs preferentially in dysplastic and neoplastic lesions, NBI may identify early dysplastic lesions better than WLB or AFB. Early studies with NBI in high-risk patients demonstrated its ability to detect lesions that could not be visualized by WLB, with a similar sensitivity to AFB.24,25 A recent study compared WLB, AFB, and NBI in the same patients who presented for airway surveillance and revealed similar sensitivity for AFB and NBI, but improved specificity with NBI for detecting abnormal lesions.24 Although current clinical applications for AFB and NBI are limited, they may play a role in future risk stratification, prognostication, or chemoprevention trials in high-risk patients.
Another promising technique, optical coherence tomography (OCT), is analogous to ultrasound imaging except that infrared light waves, rather than acoustic waves, are used.26–31 By using light instead of sound waves, OCT overcomes the major limitations of ultrasound in the lung: The inability to image through air and its poor spatial resolution. At present, OCT can resolve structures as small as 3 μm, rendering this imaging technique superior to conventional CT or magnetic resonance imaging for detecting microscopic airway abnormalities. The ability to acquire such precise views in real time may have important clinical implications in the near future.32,33 A similar modality, fibered confocal fluorescence microscopy (FCFM) is based on confocal microscopy that allows thin section imaging via use of a flexible fiberoptic miniprobe that can be introduced through a fiberoptic bronchoscope. This technology does not rely on light reflectance as in OCT, but rather cellular and tissue autofluorescence upon laser excitation. This technique may offer the possibility of an “optical biopsy” of peripheral lung lesions in the future (Video 35-3).
Video 35-3 This video demonstrates the findings on “alveoloscopy” using a confocal microscopy probe (Cellvizio™, Mauna Kea Technologies, Paris, France). A thin probe is advanced into the distal lung parenchyma through the working channel of a flexible bronchoscope and is able to image autofluorescence of structures of the lung. In particularly, elastin in the alveolar wall is readily detected, and therefore allows for visualization of the architecture of the alveolus, including areas of breakdown in the alveolar wall as may be seen in emphysema. In addition, intra-alveolar macrophages emit significant autofluorescence and are therefore readily visualized with the Cellvizio™ probe. These are the cells seen to be mobile within the elastin-containing alveolar walls on the video. Type I and type II pneumocytes are not readily visualized with this technology. Access at www.fishmansonline.com
 EVALUATION OF PERIBRONCHIAL STRUCTURES
EVALUATION OF PERIBRONCHIAL STRUCTURES
The trachea and bronchi are surrounded by mediastinal and parenchymal structures. Developmental or pathological changes in these organs may be noted during bronchoscopic evaluation. An enlarged goiter or thymus can compress upper airways, resulting in airflow obstruction. Lymphadenopathy may produce structural changes, including widening of the carina due to subcarinal involvement and compression of other bronchi—as, for example, in the right middle lobe syndrome. Calcification of peribronchial lymph nodes may result in erosion of the bronchial wall and formation of a broncholith. These lesions are potential sources of obstruction, infection, or dangerous hemoptysis.
Development of the techniques of standard TBNA and EBUS-TBNA provide diagnostic options for the evaluation of peribronchial structures that pose much less risk and a lower complication rate than mediastinoscopy; in addition, they are less costly.
 PERFORMANCE OF BRONCHIAL AND PARENCHYMAL BIOPSIES
PERFORMANCE OF BRONCHIAL AND PARENCHYMAL BIOPSIES
Improvements in bronchoscopic instrumentation since the days of Chevalier Jackson have permitted performance of endobronchial biopsies, as well as biopsy of peripheral lung lesions. Knowledge of the underlying disease process has a significant influence on the choice of specific diagnostic procedures and risk of complications. In the case of diffuse lung diseases, such as sarcoidosis, use of fluoroscopy has not been demonstrated to improve the diagnostic yield of transbronchial biopsies (TBBs). Fluoroscopy is useful, however, in providing information regarding the proximity of the forceps to the pleura and in more rapidly establishing the diagnosis of complications (e.g., pneumothorax).
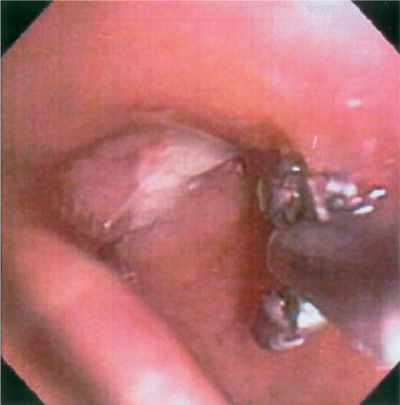
Bronchoscopically visible lesions are generally biopsied with minimal risk; if bleeding occurs, it can usually be controlled easily (Fig. 35-4). The diagnostic yield of bronchoscopy for peripheral lesions depends on a number of factors, including lesion size, its location in the lung, and on the relationship between the lesion and bronchus. The presence of a bronchus sign on chest CT predicts a much higher yield of bronchoscopy for peripheral lung lesions. In these cases, fluoroscopy is mandatory to assure proper positioning of the cytology brush, biopsy forceps, or needle. An exciting new area is the potential application of radial EBUS in evaluation of peripheral pulmonary nodules. Radial probe EBUS allows for acquisition of diagnostic tissue via TBB performed with fewer passes; it may permit differentiation between benign and malignant nodules based entirely on nodule architecture. In the future, peripheral EBUS nodule characterization may even obviate the need for pathological diagnosis in certain patients with suspicious nodules.
Figure 35-4 “Hot” forceps biopsy of a vascular endobronchial lesion. Use of the electrocautery forceps allows for safe, hemostatic biopsy of friable or vascularized endobronchial lesions (such as bronchial carcinoids), while obtaining pathologically interpretable tissue biopsy specimens.
The diagnosis of various infectious diseases can be established using a variety of transbronchoscopic sampling techniques. The role of bronchoscopic biopsy has been reaffirmed in immunocompromised hosts, in whom documentation of the precise pathogen is crucial for appropriate therapy. For example, while the presence of cytomegalovirus (CMV) in bronchoalveolar lavage (BAL) fluid may not be diagnostic, documentation of intracellular inclusion bodies on a biopsy specimen is practically pathognomonic. Simple, cost-effective transbronchoscopic tissue sampling can obviate much more complicated, expensive, and higher-risk thoracic surgical procedures.
 SAMPLING OF AIRWAY AND ALVEOLAR CONSTITUENTS
SAMPLING OF AIRWAY AND ALVEOLAR CONSTITUENTS
Bronchoscopy provides easy and relatively safe access to material in the tracheobronchial tree and distal alveolar spaces. A variety of studies are routinely performed on specimens obtained from the airways and alveolar spaces using several techniques. For example, aspirated secretions can be sent for microscopy and culture to determine the offending organism in cases of infection or suspected infection. Cytological analysis of bronchoscopically obtained materials can provide proof of malignancy. With the advent of lung transplantation, the success of the procedure depends, in large measure, on the early diagnosis of rejection or infection in these immunocompromised subjects. The most commonly employed bronchoscopic techniques for sampling the airways and alveolar spaces include “bronchial washing,” bronchial brushing, and BAL.
 BRONCHOALVEOLAR LAVAGE
BRONCHOALVEOLAR LAVAGE
A very useful bronchoscopic technique is BAL.34,35 BAL is safe, even in critically ill patients, when biopsy or brushings may be contraindicated because of the risk of bleeding. Normal saline solution, devoid of any bacteriostatic material, is instilled into distal air spaces through the “wedged” bronchoscope and then aspirated through the instrument’s suction channel (Fig. 35-5). The fluid collected in this manner is analyzed for gross appearance to detect possible alveolar hemorrhage. The fluid may also be subjected to a variety of tests, depending on the clinical circumstances: Microbiological testing, specific cytological analysis and cell count, immunological parameters, presence of various biochemical mediators related to pathological processes, tissue markers, polymerase chain reaction, electron microscopy, flow cytometry, and DNA probes.
Figure 35-5 Bronchoalveolar lavage is performed by wedging the tip of bronchoscope in the segmental bronchus of interest. Normal saline is instilled into the distal air spaces, and then collected by suctioning back into a sterile container.
Overall, the diagnostic yield of BAL is very much dependent on specific patient characteristics, underlying pathological process, and many technical factors.
INDICATIONS FOR DIAGNOSTIC BRONCHOSCOPY
Although there are several indications for diagnostic bronchoscopy (Table 35-1), evaluation of a lung nodule or mass and mediastinal staging of a lung cancer are the most common. There are many other potential indications, some of which are discussed subsequently.36
Source: Reproduced with permission from Casal RF, DE Ost, GA Eapen. Flexible Bronchoscopy. Clin Chest Med. 2013;34(3):341–352.
 BRONCHOGENIC CARCINOMA
BRONCHOGENIC CARCINOMA
Bronchoscopy plays a central role in the evaluation of lung masses and nodules, including those suspicious for bronchogenic carcinoma.
Diagnosis
Bronchoscopy most commonly is performed in the evaluation of patients with suspected lung cancer. It remains the most commonly used modality for the diagnosis of bronchogenic carcinoma and plays an important role in staging of the disease, as well. Centrally located lesions generally may be approached using flexible bronchoscopy with minimal risk. Bronchogenic carcinoma of the central airways may manifest as exophytic mass lesions with partial or total bronchial lumen occlusion, as peribronchial tumors with extrinsic compression of the airway, with submucosal tumor infiltration, or with some combination of these entities. The mucosal abnormalities seen with peribronchial tumors or with submucosal infiltration often are subtle—the airways should be examined closely for characteristic changes such as erythema, loss of bronchial markings, and nodularity of the mucosal surface.
Central lesions usually are sampled using a combination of bronchial washes, bronchial brushings, and endobronchial biopsies. The yield of bronchoscopy is highest for endoscopically visible lesions, with a diagnostic yield of approximately 90%.37 Attempts should be made to obtain biopsy specimens from areas of the lesion that seem viable. Endobronchial needle aspiration (EBNA) to obtain a “core” biopsy from centrally located tumors should be considered, particularly if the lesion appears necrotic (Video 35-4). For submucosal lesions, EBNA can be performed by inserting the needle into the submucosal plane at an oblique angle; in patients with peribronchial disease causing extrinsic compression, the needle should be passed through the bronchial wall into the lesion. For all of these indications, EBNA has been shown to increase the diagnostic yield over conventional sampling methods.
Video 35-4 Demonstrated in this video is the utilization of a standard 22-gauge Wang™ transbronchial aspiration needle (MW-122, ConMed, Utica, NY) for sampling of a partially necrotic endobronchial lesion in the right main stem bronchus. The advantages of bronchoscopic needle aspiration of endobronchial lesions include decreased bleeding risk compared to endobronchial biopsy or brushing; facilitation of sampling of material from the center of the lesion avoiding necrotic surface; and the ability to obtain real-time feedback through rapid on-site evaluation (ROSE) of aspirates by cytopathology. Access at www.fishmansonline.com
Evaluation of Peripheral Lesions and Lung Nodules
The evaluation of peripheral lesions or lung nodules remains a common dilemma for chest physicians. A balance between pretest probability of a specific diagnosis and the complication risk associated with a biopsy method must be assessed.38 Surgical biopsy offers a superior yield at the cost of increased cost and morbidity. TTNB has a high yield, but it carries a 15% to 25% risk of pneumothorax.39–41 Although bronchoscopy has the advantage of a low complication risk, it has been hampered by a significantly lower diagnostic yield than these other modalities.
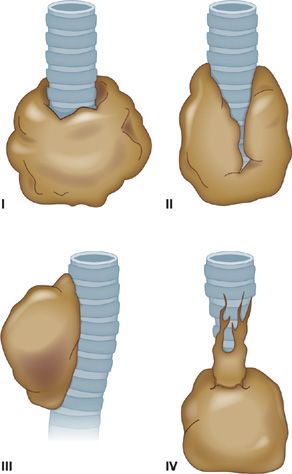
The yield of flexible bronchoscopy for peripheral pulmonary lesions, defined as lesions that are not visible beyond the segmental bronchi, is significantly lower than for central lesions. The overall sensitivity of standard bronchoscopy, based on studies that used a combination of TBB, cytology brush, BAL, and TBNA is 78% for peripheral disease.42–58 Success depends on several factors, including lesion size, distance from the proximal airways, and the presence of a bronchus sign. The bronchus sign on CT may reflect the relationship of a tumor with the airway, and it has been categorized into four patterns by Tsuboi59: type I, in which the bronchial lumen is patent up to the tumor; type II, in which the bronchus is contained in the tumor mass; type III, in which the bronchus is compressed, narrowed, and displaced by the tumor, but the bronchial mucosa is intact; and type IV, in which the proximal bronchus is narrowed by the submucosal and peribronchial spread or tumor or by the enlarged lymph nodes (Fig. 35-6). TBB has the lowest diagnostic yield for lesions with a type III or IV tumor–bronchus pattern. In these cases, the use of peripheral TBNA may improve the overall diagnostic yield of bronchoscopy.
Figure 35-6 Tsuboi classification of tumor–bronchus relationship. (see text for details)
A recent study evaluating the use of conventional diagnostic bronchoscopy for screen-detected nodules demonstrated a diagnostic yield of only 13.5%, with a negative predictive value of 47.6%.60 Given this poor performance, conventional bronchoscopy should not be performed in the evaluation of small, peripheral pulmonary nodules.39,54 The use of guided bronchoscopic approaches (e.g., navigational bronchoscopy, UM-EBUS, or ultrathin bronchoscopy) has significantly improved the diagnostic yield of small, peripheral pulmonary lesions. In a randomized comparison of traditional, fluoroscopically guided TBBs versus UM-EBUS–guided TBB, no statistical difference was found in establishing a diagnosis for lesions greater than 3 cm; however, for lesions smaller than 3 cm and for lesions smaller than 2 cm, the sensitivity of EBUS-guided TBB remained at 75% and 71%, whereas that of standard TBB fell dramatically to 31% and 23%, respectively.61 Similar improvements have also been reported with the use of EMB- or VB-based systems for peripheral pulmonary lesions.
A recent meta-analysis of 30 studies with >3000 nodules reported a combined diagnostic yield of 70% for all available guided bronchoscopic techniques.62 The diagnostic yield was influenced by the size of the primary lesion, with a yield of 61% for lesions <2 cm and 82% for lesions >2 cm.62 Several other factors have also been described that impact the likelihood of obtaining diagnostic tissue using guided bronchoscopy: Ability to place UM-EBUS probe completely within a lesion (as opposed to adjacent to it), presence of a bronchus sign on CT scan, location in the middle lobe or lingula, and distance from the visceral pleura.63–66 The use of TBNA in addition to conventional diagnostic procedures (TBB and BAL) in UM-EBUS–localized lesions also significantly improves diagnostic yield.67
Although it is increasingly clear that guided approaches are better than conventional bronchoscopy for smaller lesions, there are few comparative studies guiding the selection of a particular modality. In one randomized study of EMB alone, UM-EBUS alone, or EMB followed by confirmatory UM-EBUS, there was a significantly higher yield with the combined approach (combination, 88%; UM-EBUS, 69%; EMN, 59%),68 suggesting that the highest yield may come from combined diagnostic modalities. However, a recent randomized study comparing ultrathin bronchoscopy with and without VB did not demonstrate any significant difference in the overall diagnostic yield (67% vs. 60%), although VB-assisted bronchoscopy was better for lesions in the RUL, lesions invisible on plain films, and lesions in the peripheral third of the lung.69 Further studies are needed to help define the utility and specific indications for combination approaches for the diagnosis of peripheral lung lesions.
Staging
Bronchoscopy is an important modality for establishing lung cancer stage. In patients with potentially resectable tumors, a thorough airway examination helps confirm the absence of a concomitant, radiographically occult lesion. For lesions that involve the central airways, it is important to document the extent of disease and the degree of involvement of the main stem bronchi and main carina.
The use of CP-EBUS allows for real-time visualization and TBNA of hilar and mediastinal lymph nodes (Video 35-5). Sampling of lymph nodes using EBUS-TBNA is now commonly performed for nodal staging in patients with lung cancer. This technique frequently allows the establishment of both diagnosis and nodal stage, obviating the need for, and avoiding the risks associated with, sampling of the primary parenchymal lesion. TBNA has proved particularly useful with the employment of rapid on-site evaluation (ROSE), in which a cytopathologist present in or near the bronchoscopy suite can evaluate specimens in real time.
Video 35-5 This video demonstrates use of a 22-gauge EBUS-TBNA aspiration needle (Olympus, Center Valley, PA) to sample a left hilar node. The convex probe-EBUS scope is used to identify the left hilar node, allowing visualization of the TBNA needle entering the node in real time. The TBNA needle can be seen entering the left hilar node from the upper left portion of the video. The to-and-fro needle movement allows for collection of lymph node aspirate material. Access at www.fishmansonline.com
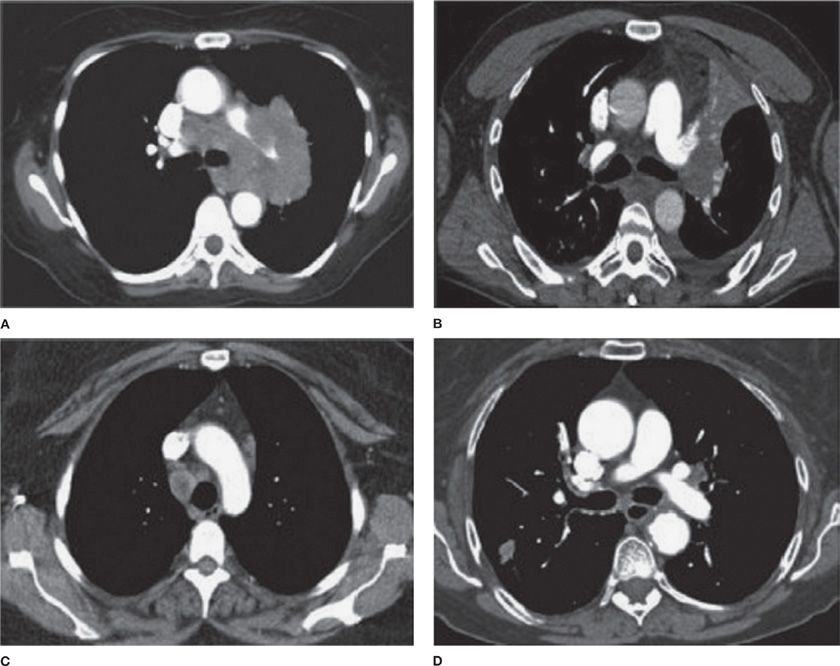
In general, patients with lung cancer may be separated into four categories with respect to intrathoracic radiographic characteristics, as suggested by the American College of Chest Physicians (ACCP) guidelines on lung cancer staging (Fig. 35-7).70 In radiographic pattern A, mediastinal infiltration is extensive, with encircling of vessels and airways. In this case, the risk of malignant involvement can be assumed, and biopsy to establish diagnosis should be performed by the safest method available for that particular case. In pattern B, there is discrete lymph node enlargement that can be measured by CT, which may also be FDG-avid. In this case, sampling is recommended as the likelihood of mediastinal involvement is high and requires confirmation. In radiographic pattern C, the presence of a central tumor or suspected N1 disease makes the likelihood of mediastinal node involvement relatively high (20%–25%), necessitating invasive sampling. In the final group (i.e., those with a peripheral clinical stage I tumor), the chance of either distant metastases or mediastinal involvement is quite low (radiographic group D), especially if the mediastinum is also normal by positron emission tomography (PET) scan. The decision to pursue invasive staging should be considered on an individual case basis.
Figure 35-7 American College of Chest Physicians intrathoracic radiographic (CT scan) categories of lung cancer. A. Mediastinal infiltration by tumor. B. A central tumor or a tumor with enlarged N1 nodes, but a normal mediastinum. C. Enlarged discrete N2,3 nodes. D. A peripheral small tumor (seen in lower left corner of image) with normal-sized lymph nodes. (Reproduced with permission from Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed. American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e211S–250S.)
Several systematic reviews have confirmed that the sensitivity of EBUS-TBNA is equivalent to that of mediastinoscopy.70–73 A recent randomized study of 241 patients with potentially resectable NSCLC compared mediastinoscopy alone to a combined approach of EBUS-TBNA and EUS-FNA, followed by surgical staging if the needle technique did not identify nodal metastases.74 The needle approach followed by mediastinoscopy resulted in a significantly greater sensitivity (94%) compared to the other approaches and resulted in fewer unnecessary thoracotomies. One limitation to this approach has been the difficulty in many centers to operationalize a combined EBUS-TBNA and endoscopic ultrasound (EUS)-FNA procedure. Interestingly, recent studies have shown that the EBUS-TBNA scope can be used through both the airway and the esophagus, resulting in similar results.75,76 The ACCP guidelines now recommend that a needle approach should be used first when performing mediastinal staging. However, because of a relatively high false-negative rate, a negative result with a needle technique should prompt consideration of surgical staging methods.
 EVALUATION OF HEMOPTYSIS
EVALUATION OF HEMOPTYSIS
One of the most frequent indications for bronchoscopy is hemoptysis. Bronchoscopic evaluation can be of help in determining the precise location and source of bleeding. The choice of instrument (rigid vs. flexible scope) and timing of the procedure are dictated by clinical circumstances.77 Studies have shown that active bleeding and its site are visualized more commonly with early bronchoscopy (within 48 hours) than with more delayed examination.77–79 In the case of a normal chest radiograph and hemoptysis, trace signs of bleeding are commonly seen, but not the site of origin.80 In these circumstances, examination using an ultrathin flexible instrument may be beneficial in identifying the source of bleeding in a peripheral airway once the more proximal airways have been cleared of blood by a therapeutic scope. In some instances, bronchoscopy is useful not only as a diagnostic method, but also to perform therapeutic maneuvers (see Chapter 36, Interventional Bronchoscopy).
 PULMONARY INFECTIONS
PULMONARY INFECTIONS
Bronchoscopy is a useful technique in the diagnosis of pulmonary infections, allowing for the collection of respiratory samples for evaluation with special stains and culture. Several common clinical areas in which bronchoscopy may play an important diagnostic role are described subsequently.
Pneumonia
In general, bronchoscopy is not indicated for the diagnosis of community-acquired pneumonia, which is currently treated empirically with appropriate antibiotic therapy; however, bronchoscopy is likely to be useful in cases of nonresolving pneumonia,81,82 defined as a lack of improvement or worsening of symptoms despite a minimum of 10 days of antibiotic therapy or failure of radiographic abnormalities to resolve after 2 to 3 months. The causes of nonresolving pneumonia are myriad and include inadequate antibiotic therapy, resistant or highly virulent organisms, impaired host defenses, obstructing endobronchial lesions, or a noninfectious cause. Although controversial, bronchoscopy should be considered in these patients.
Ventilator-Associated Pneumonia
Ventilator-associated pneumonia (VAP) is defined as a pneumonia occurring more than 48 hours after intubation and initiation of mechanical ventilation.83,84 VAP is usually suspected when an intubated, mechanically ventilated patient has signs of infection and an abnormal chest radiograph. Intubated patients experience colonization of their upper and lower airways with nosocomial organisms; because of an abnormal mucociliary clearance mechanism, these patients are at greater risk for developing pulmonary infections. In addition, mechanically ventilated patients are often treated empirically with broad-spectrum antibiotics and, therefore, are at greater risk for infection with resistant organisms and unusual lower respiratory tract pathogens. Guidelines support the use of either a quantitative or semiquantitative strategy in the diagnosis of VAP.83 Quantitative sampling can be performed using endotracheal aspirates, or BAL or protected specimen brush (PSB) collected with or without a bronchoscope.83,85
Quantitative BAL entails the performance of a standardized BAL, with infusion of at least 120 mL of saline for adequate sampling of a pulmonary subsegment. Quantitative culture of the aspirated material is performed to determine the number of colony-forming units (CFUs) recovered.
PSB uses a double catheter system in which an outer cannula and distal, biodegradable plug protect the bronchoscopic brush within the inner cannula from contamination with secretions in the upper airway and suction channel of the bronchoscope. When the bronchoscope is positioned proximal to the segmental orifice of interest, the PSB inner cannula is advanced into a subsegment and the protective distal plug ejected. The brush is then advanced peripherally, rotated gently, and retracted into the inner cannula. The inner cannula is subsequently retracted into the outer cannula and the bronchoscope removed from the airway. The distal portion of the catheter is cleaned with 70% alcohol and the brush clipped into saline solution under sterile conditions. The PSB is then submitted for quantitative bacterial culture within 15 minutes of performance of the procedure.
The threshold for diagnosis of VAP using PSB is 103 CFU/mL. PSB has higher specificity than sensitivity for the presence of VAP—a positive result greatly increases the likelihood that pneumonia is present. For quantitative BAL, a threshold of 104 or 103 CFU/mL is used for diagnosis of VAP.86 The detection of VAP by quantitative BAL culture has a sensitivity of 40% to 90% and a specificity of 45% to 100%.86 Because a larger proportion of lung parenchyma is sampled with BAL, this may be a better method than PSB for VAP diagnosis. However, samples contaminated by upper airway secretions (based on a high percentage of squamous epithelial cells) should be interpreted with caution. There are likely a number of factors that affect the diagnostic yield of the bronchoscopic methods, such as a change in antibiotics before sampling, inadequate technique in sampling, and lack of a gold standard for comparison.
Infections in Immunocompromised Patients
Pulmonary infections in immunocompromised patients constitute the most common complication in this population and represent an important contributor to mortality. Such infections are increasingly common, reflecting the expanding use of aggressive chemotherapeutic regimens and the ever increasing number of solid organ and hematopoietic stem cell transplantations. The differential diagnosis of pulmonary infiltrates is broad in scope; however, most cases are caused by infectious agents, including bacterial, fungal, viral, and mycobacterial pathogens.87 Bronchoscopy is the most commonly used diagnostic procedure in these patients and should be performed as early as possible, because a delay in diagnosis of longer than 5 days has been shown to significantly increase mortality.
The sensitivity of bronchoscopy varies, depending on the immunocompromised population studied and the specific etiological disorder. In non–human immunodeficiency virus (HIV)-infected patients, the yield of BAL for Pneumocystis jirovecii pneumonia (known previously as Pneumocystis carinii pneumonia [PCP]) is approximately 80%, compared with a greater than 90% yield observed in HIV-seropositive patients.88,89 This difference is due to the much lower organism load present in non–HIV-seropositive subjects. Although empirical therapy often is initiated in patients suspected of having PCP infection, bronchoscopy should be performed in most cases to confirm the diagnosis. Bronchoscopic lung biopsy may increase the diagnostic yield of BAL for diagnosis of PCP infection, particularly in the non–HIV-infected population.88 Bronchoscopy also has a high diagnostic yield for CMV; however, because CMV cultures from BAL are not specific, the diagnosis of CMV pneumonia should be limited to patients with pathological evidence of CMV infection demonstrated by the presence of CMV inclusion bodies on BAL or biopsy. Although bronchoscopy also is useful for the diagnosis of aspergillosis – the sensitivity is approximately 50% – the disease often is peripheral and patchy and, thus, is not easily diagnosed by BAL or bronchoscopic biopsy. Overall, in immunocompromised patients with infiltrates, the diagnostic yield of bronchoscopy varies from 30% to 80% and is impacted by factors such as the prevalence of an infectious etiology, timing of bronchoscopy, and use of prophylactic antibiotics.90–97
Mycobacterial Infections
In cases in which pulmonary tuberculosis is suspected, the initial diagnostic evaluation should consist of serial examination of sputum for the presence of acid-fast bacilli in stained smears. Ideally, induced sputum samples should be obtained. If sputum study results are negative, or if a patient is unable to produce sputum and tuberculosis is still suspected, bronchoscopy with BAL and biopsy should be performed. The use of bronchoscopy allows for the opportunity to establish a rapid diagnosis (by positive smear or histopathology), providing the potential for earlier intervention and treatment while awaiting culture results. Bronchoscopy should be performed with appropriate infection control precautions to minimize the risk of nosocomial transmission. A bronchoscopy may cause the patient to produce sputum for several days afterward; these specimens also should be collected and analyzed, if possible.
The utility of bronchoscopy in establishing a rapid diagnosis varies widely in the literature, with reported diagnostic yields of 30% to 70%, although the overall yield of culture is considerably higher.98 One study has shown an improvement in diagnostic yield from 58% to 81% with use of UM-EBUS–guided biopsy and washings.99 The yield in patients with miliary tuberculosis, in whom sputum smears frequently are negative, is approximately 70%. Bronchoscopy also is useful in tuberculosis manifesting as an endobronchial lesion or with mediastinal and hilar adenopathy, in which case, diagnostic tissue may be obtained with TBNA.
Human Immunodeficiency Virus Syndrome
The introduction of highly active antiretroviral therapy (HAART) has resulted in a sharp decline in the incidence of opportunistic infections in HIV-infected patients. Nevertheless, infectious complications remain one of the most common indications for bronchoscopy in this population. PCP remains the most frequent serious opportunistic infection in HIV-seropositive patients. Bronchoscopy with BAL remains the preferred diagnostic procedure for this disease, although in select centers, use of sputum induction has had a relatively high diagnostic yield and may mitigate the need for bronchoscopy. As previously mentioned, bronchoscopic lung biopsy may increase the diagnostic yield of BAL.88 Empirical therapy often is initiated in patients with suspected Pneumocystis infection; such therapy can impair the diagnostic yield of BAL if the procedure is not performed within 24 hours. In patients receiving pentamidine prophylaxis, the diagnostic yield is decreased unless the upper lobes are sampled.100–102 Several PCR assays have been tested on BAL fluid, induced sputum, and oral wash specimens; these generally have been more sensitive, but less specific, than traditional microbiological methods.
Bronchoscopy also plays an important diagnostic role in HIV-positive patients with infections caused by mycobacteria, including tuberculosis, atypical bacterial pneumonias, and various fungal infections. Kaposi sarcoma, caused by human herpesvirus type 8 (HHV8), can manifest with violaceous endobronchial plaques that typically occur at airway bifurcations; pulmonary parenchymal involvement is characterized by lymphangitic infiltration of tumor, leading to the development of nodules and masses.
 DIFFUSE LUNG DISEASES
DIFFUSE LUNG DISEASES
A wide range of acute and chronic pulmonary disorders are capable of causing a diffuse interstitial lung disease pattern of injury. These processes include infection, neoplasm, pulmonary edema, alveolar hemorrhage, alveolar proteinosis, occupational lung diseases, drug-induced disease, and various types of idiopathic or collagen vascular disease–associated interstitial lung disease. The pattern of lung injury should first be evaluated using high-resolution chest CT imaging, which helps to narrow the differential diagnosis and, in some cases, is virtually diagnostic of certain disorders. In many cases, it is still necessary to obtain samples for cytological and histological evaluation to confirm a specific diagnosis and to help exclude other possible disorders.
The most common bronchoscopic procedures used to help establish the diagnosis in diffuse lung disease are BAL and TBB. The findings on high-resolution CT (HRCT) can be used to determine the best location for BAL or TBB. In truly diffuse disease, the right middle lobe and the lingula are the best locations for BAL; with these sites, ease of access and good fluid retrieval are typical. BAL should be performed using a total of 100 to 200 mL of saline instilled in multiple aliquots. It is important to obtain a reasonable sampling of the alveolar spaces for the necessary cellular analysis.
The value of BAL is well documented in the diagnosis of diffuse parenchymal diseases, and findings may be diagnostic in eosinophilic pneumonia, eosinophilic granuloma, and pulmonary alveolar proteinosis.36 In most other diffuse disorders, BAL findings are mostly supportive of a suspected diagnosis (Table 35-2) and helps to rule out potential infectious etiologies. In disorders such as sarcoidosis, hypersensitivity pneumonitis, and organizing pneumonia, the use of BAL in combination with bronchoscopic lung biopsy can often establish the diagnosis and avoid the need for surgical lung biopsy. For example, with pulmonary sarcoidosis, the diagnosis usually is established by a combination of BAL and biopsy findings. The BAL can be used to exclude the presence of tuberculosis and fungal infections and may demonstrate the characteristic high CD4+/CD8+ ratio seen in sarcoidosis, whereas bronchoscopic biopsy specimens may demonstrate the classic finding of noncaseating granulomas. In general, TBB should be performed in several affected areas, and at least five or six specimens should be taken. The sensitivity of TBB for diagnosis of sarcoidosis is only approximately 60% to 70%, and many patients require further invasive testing, such as surgical lung biopsy.103,104