Diagnosis and Treatment of Pain, Agitation, and Delirium in the Intensive Care Unit
INTRODUCTION
The large majority of patients in intensive care units (ICUs) experience pain, agitation, or delirium at some point during their critical illness. During the last two decades, tremendous growth in knowledge regarding the assessment and management of these syndromes in the ICU has prompted important changes in the practice of intensive care medicine. Validated tools now exist that allow clinicians to quickly and reliably evaluate patients for pain, assess their level of consciousness, and detect delirium. These assessments, in turn, guide clinicians as they choose and titrate therapies targeted to best manage a patient’s symptoms. Novel strategies to address pain, agitation, and delirium have also recently been investigated. The use of validated tools to diagnose these important clinical issues using evidence-based management strategies has been shown to improve both short- and long-term outcomes in numerous studies. This chapter reviews these assessment tools and presents best practices for management in critically ill patients.
PAIN IN THE CRITICALLY ILL
Critically ill patients frequently suffer from pain.1,2 Whereas effective treatments for pain are widely available, detecting pain in those critically ill patients who are unable to report it is challenging. Prolonged pain in the critically ill is associated with adverse physiologic effects.3 Because analgesia is most effective when pain is identified and treated early, clinicians should frequently assess patients for pain and treat it when identified.4
 ASSESSING PAIN IN THE CRITICALLY ILL
ASSESSING PAIN IN THE CRITICALLY ILL
Pain is subjective in nature; therefore, a patient’s self-report (using numeric scale or visual analog scale) is an easy and reliable method to determine its presence. In fact, the 2013 Society of Critical Care Medicine (SCCM) clinical practice guidelines on the management of pain, agitation, and delirium in the ICU suggest that the numeric rating scale be used to assess pain when patients are able to self-report.5 Among the critically ill, however, self-reporting is not always possible due to the presence of endotracheal tubes, sedation, or delirium. Therefore, several instruments have been developed and validated to aid clinicians in identifying the presence of pain in critically ill patients.
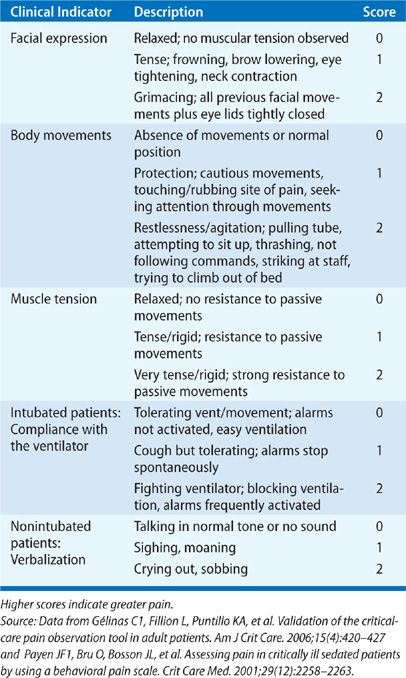
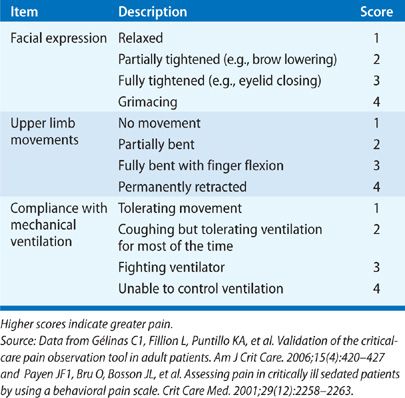
The two most widely studied pain assessment tools for use in patients in the ICU who are unable to report pain are the Behavioral Pain Scale (BPS) and the Critical Care Pain Observation Tool (CPOT).6,7 Both the BPS and CPOT utilize nonverbal cues and patient behaviors commonly indicative of the presence of pain; these include facial expressions, body movements, and compliance with mechanical ventilation to establish the presence of pain (Table 150-1A,B). Alternatively, a patient’s surrogate may be able to provide description of behaviors indicative of pain in a particular patient, based on the surrogate’s prior knowledge of the patient.8 Finally, in the case where the clinician is unsure if a patient is having pain, a trial of an analgesic medication can be used to assess for a decrease in the suspected pain-related behaviors.8 Regardless of the assessment method used, it is important to note that assessing for pain among the critically ill is associated with improved clinical outcomes, including optimized analgesic and sedative administration and reduced duration of mechanical ventilation and ICU stay.9–11
 TREATING PAIN IN THE CRITICALLY ILL
TREATING PAIN IN THE CRITICALLY ILL
Comprehensive pain management in the critically ill consists of pharmacologic therapies (e.g., opioid medications and nonopioid medications) in combination with nonpharmacologic approaches to ensure adequate analgesia.4
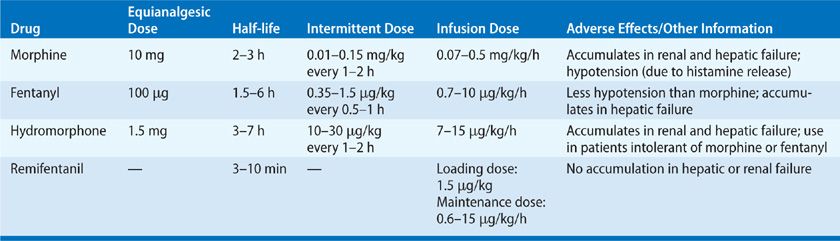
First-line treatment for acute, nonneuropathic pain among the critically ill is intravenous opioid analgesics, such as fentanyl, morphine, hydromorphone, methadone, and remifentanil. The specific drug choice and dose are determined by the severity of the pain, patient characteristics (e.g., age, renal, and hepatic function), the patient’s clinical status, and the properties of the drug (e.g., pharmacokinetics and pharmacodynamics) (Table 150-2).
Nonopioid analgesics (e.g., acetaminophen, ibuprofen, ketamine) may be administered as adjuvant therapies to reduce the overall dose of opioids. Nonpharmacologic interventions such as massage, relaxation techniques, heat or ice application, and music therapy may provide additional analgesic effect.4 For patients experiencing neuropathic pain, use of gabapentin or carbamazepine may be added to opioid and nonopioid analgesics.5
AGITATION IN THE CRITICALLY ILL
Agitation in critically ill patients is associated with adverse outcomes, such as the removal of life-support devices and danger to the patient or beside staff. Commonly, agitated patients are given sedatives to reduce agitation and prevent harm.12 Nevertheless, it is important to remember that agitation does not equate to “sedation deficiency.” Rather, the presence of agitation should prompt the clinician to search for and treat an underlying cause, such as pain, hypoxemia, metabolic derangements, developing sepsis, delirium, or withdrawal from alcohol or other drugs. Agitation exists at one extreme of the continuum of consciousness that also includes calm states and states of decreased arousal, such as stupor and coma. This section reviews methods to assess level of consciousness, as well as best practices for the treatment of agitation, primarily through the judicious use of sedative medications.
 ASSESSING AGITATION AND LEVEL OF CONSCIOUSNESS IN THE CRITICALLY ILL
ASSESSING AGITATION AND LEVEL OF CONSCIOUSNESS IN THE CRITICALLY ILL
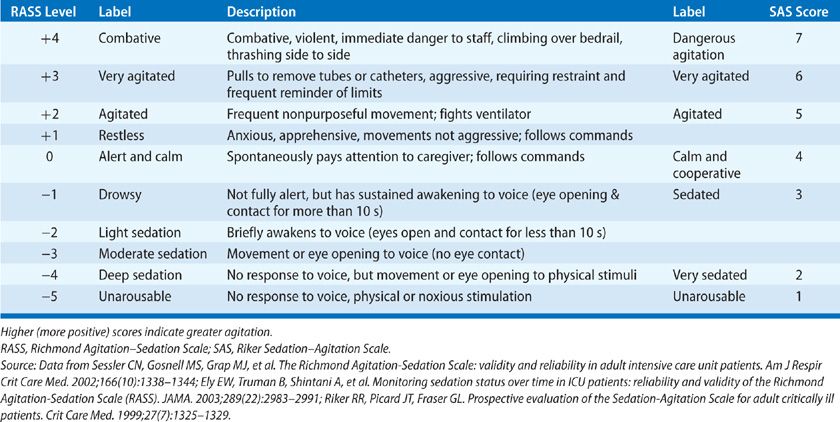
A number of standardized measures can be used to determine level of consciousness in the critically ill. Although these tools are commonly referred to as “sedation-agitation scales,” they can be used to evaluate all patients in the ICU, regardless of whether they are receiving sedatives, as they assess patient behaviors and response to verbal or physical stimuli to determine the patient’s level of consciousness. The current SCCM guidelines recommend the Richmond Agitation–Sedation Scale (RASS) and Riker Sedation-Agitation Scale (SAS), noting that these scales are the most valid and reliable for determining level of consciousness during critical illness (Table 150-3). Unlike the Glasgow Coma Scale (GCS), the RASS and SAS have been validated for use in the setting of ongoing critical illness, even when patients are sedated or nonverbal due to placement of an endotracheal tube. Regular assessments of level of consciousness can improve detection of changes in neurologic status, as well as facilitate and guide delivery of sedatives and other psychoactive medications.
TABLE 150-3 Level of Consciousness Scales: Richmond Agitation–Sedation Scale (RASS) and Riker Sedation–Agitation Scale (SAS)
 TREATING AGITATION IN THE CRITICALLY ILL
TREATING AGITATION IN THE CRITICALLY ILL
The first step in treating agitation should be to seek out the underlying (and potentially reversible) cause(s). Providing adequate analgesia, treating hypoxemia with supplemental oxygen, correcting electrolyte abnormalities, treating infection, providing reorientation, ensuring an environment conducive to sleep, and treating alcohol and drug withdrawal may eliminate agitation in the appropriate situation. If agitation persists despite measures aimed at addressing the underlying cause, consideration should be given to administration of sedatives.
The past decade has seen a dramatic shift in the strategies used to sedate patients in the ICU. As a result of numerous trials reporting better outcomes among patients managed using protocol-based administration of sedation compared with those managed without the guidance of a protocol,13–16 an increasing number of ICUs are adopting algorithmic approaches to the delivery of sedatives to critically ill patients. While a multitude of protocols exists, common threads are present in each, including objective assessment of level of consciousness; prioritization of analgesia over administration of sedatives; targeting lighter, rather than moderate or heavy sedation, using intermittent dosing of medications or daily interruption of medications; and relying on benzodiazepine-sparing regimens.
 PRIORITIZING ANALGESIA
PRIORITIZING ANALGESIA
As discussed earlier in this chapter, pain is pervasive among critically ill patients and is a frequent reason for agitation. Thus, evaluation and treatment of pain using the techniques described earlier may be sufficient to restore a patient to a calm and alert state. Several studies of sedation protocols that preferentially treated pain before administering sedatives have demonstrated improved patient outcomes,13,17,18 and current guidelines recommend treatment of pain prior to administration of sedatives.5
A dramatic example of the value of pain management prior to administration of sedatives was provided in a randomized trial comparing a control group (sedated primarily with propofol) with patients managed using a strategy of “no sedation.”19 Patients received morphine for pain first, then haloperidol for agitation, then, if needed, intermittent short-course (i.e., 6-hour) administration of propofol. Only after failing this regimen (as indicated by ongoing agitation) did patients in the intervention group receive sedation using a more conventional propofol- and midazolam-based sedation strategy. Patients managed with the “no sedation” strategy had significantly better outcomes than those in the control group, spending more time off mechanical ventilation and experiencing earlier discharge from the ICU and hospital. Only 18% of patients managed using the “no sedation” strategy required continuous propofol >6 hours, a result that calls into question the still common assumption that endotracheal intubation and mechanical ventilation cannot be tolerated without the use of moderate or heavy sedation.
 INTERMITTENT VERSUS CONTINUOUS DOSING OF SEDATIVES
INTERMITTENT VERSUS CONTINUOUS DOSING OF SEDATIVES
As demonstrated in the aforementioned “no sedation” trial, which prioritized use of intermittent morphine and haloperidol, many analgesic and sedative medications can be delivered via intermittent (bolus), rather than continuous, dosing. The latter has been the most common method used in ICUs,9 with routine use of continuous infusions reflecting the fact that the majority of early studies of sedation in the ICU utilized continuous infusion strategies. Nevertheless, continuous infusion of analgesics and sedatives can lead to accumulation of the infused drug, resulting in delayed clearance and prolonged effects. Moreover, continuously infused medications are readily titrated to higher doses to treat agitation, but often the infusion may not be rapidly titrated to lower doses once the acute agitation has been controlled. These factors may combine to lead to adverse outcomes, including prolongation of mechanical ventilation and ICU and hospital stays.
One study addressed the association between continuous infusions and outcomes among 242 mechanically ventilated patients in the ICU.20 Patients who were managed using intermittent doses of benzodiazepines were extubated sooner, were discharged from the ICU and hospital earlier, and had lower rates of reintubation compared with those who received continuous sedation. Based on these findings, the same group developed a sedation protocol that prioritized analgesia first, then intermittent bolus sedatives, before allowing continuous infusions of sedatives. In a randomized controlled trial, this sedation protocol resulted in earlier extubation and discharge from the ICU and hospital compared with management without a sedation protocol.13 As previously described, the study comparing “no sedation” versus propofol-employed bolus doses of morphine to control pain, and even extended the preference for intermittent treatments to the use of propofol, which was limited, when possible, to only short 6-hour treatment periods—an approach that significantly improved outcomes.19
 AVOIDING BENZODIAZEPINES
AVOIDING BENZODIAZEPINES
Although early trials of sedation protocols13 utilized benzodiazepines as the sedative of choice (when an analgesia-only approach did not adequately treat agitation), more recent trials have consistently shown that benzodiazepines lead to worse outcomes than alternative sedative agents. These findings led to the SCCM guidelines suggesting that nonbenzodiazepine sedatives are preferred over sedation using benzodiazepines.
In the late 1980s and 1990s, more than a dozen randomized trials found sedation with propofol led to better outcomes than sedation with benzodiazepines.21 However, the results did not lead to changes in standard of care, in part because control groups used continuously infused midazolam rather than intermittent boluses. In fact, clinical guidelines published in 2002 recommended lorazepam as the drug of choice for sedating patients in the ICU.22 Therefore, a randomized trial was conducted to compare intermittent lorazepam with continuous propofol for the sedation of mechanically ventilated patients in the ICU.23
Patients in both groups were managed using daily interruption of sedatives. Despite the avoidance of continuous infusions in only the lorazepam group (since propofol generally requires delivery by continuous infusion when used in the ICU), patients sedated with propofol spent fewer days on mechanical ventilation. These findings were consistent with nearly every other trial comparing propofol with benzodiazepines, suggesting that continuous sedation with shorter-acting agents, such as propofol, when interrupted daily, improve outcomes compared with intermittent doses of benzodiazepines.
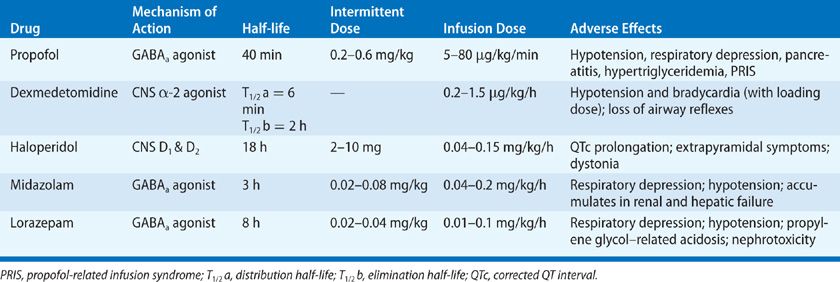
More recently, benzodiazepine-based sedation has been compared with other sedative agents; the alternatives to benzodiazepines have been found superior. Seven randomized trials compared sedation with benzodiazepines with dexmedetomidine among mechanically ventilated patients in the ICU; all seven found that patients randomized to sedation with dexmedetomidine had better outcomes, for example, less delirium and earlier extubation, than those sedated with benzodiazepines.24–30 Similarly, in three trials randomizing mechanically ventilated patients to remifentanil-based sedation versus benzodiazepine-based sedation, those sedated with benzodiazepines had worse outcomes.31–33 The pharmacologic properties of these and other agents commonly used for sedation in the ICU are presented in Table 150-4.
 TARGETING LIGHT SEDATION AND DAILY INTERRUPTION OF SEDATIVES
TARGETING LIGHT SEDATION AND DAILY INTERRUPTION OF SEDATIVES
Regardless of which medications are used to provide analgesia and sedation, it should be given in the smallest doses necessary to treat pain and agitation while allowing patients to maintain wakefulness, that is, light, rather than heavy, sedation should be the goal. Sedation protocols not only prioritize analgesics and intermittent sedatives over continuous infusions, they may also target light sedation (measured using a sedation scale) over moderate or heavy sedation.13 Other studies34 that randomized mechanically ventilated patients to targeted light versus moderate or heavy sedation also found that those managed with light sedation had shorter periods of ventilator dependency and ICU stays, as well as fewer disturbing memories of the ICU, compared with those managed with heavier sedation.
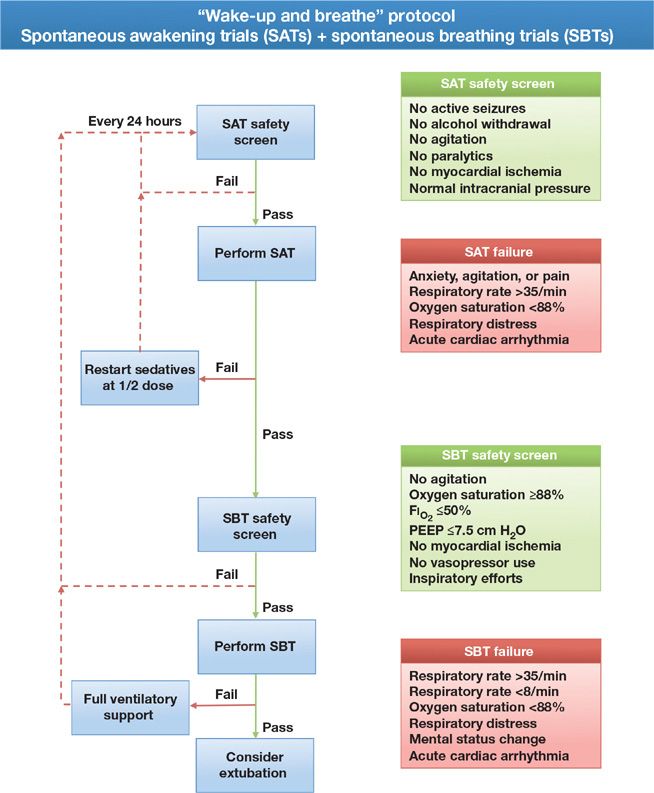
Whenever given, sedatives should be held each day that basic safety criteria are met, so that the clinician may assess the need for, and optimal dose of, analgesic and sedative medication required to maintain patient comfort and alert mental status. This period of daily interruption of sedatives, also referred to as a daily “wake-up,” “sedation holiday,” or “spontaneous awakening trial,” led to significantly better outcomes in two randomized trials. In one,14 patients managed with daily interruption of sedatives had shorter durations of mechanical ventilation and shorter stays in the ICU, and in another,15 those managed using this strategy were not only extubated earlier than those in the control group, they also were discharged earlier from the ICU and hospital; furthermore, their 1-year mortality was 14% lower than that observed in the control group. The latter trial paired daily interruption of sedatives or daily spontaneous awakening trials with daily spontaneous breathing trials as part of a strategy referred to as “Wake-up and Breathe” (Fig. 150-1).
Figure 150-1 Wake-up and breathe flowchart. Each day patients should be assessed for safety criteria to perform a spontaneous awakening trial (SAT), if the safety screen is passed, all sedating medications are held until the patient is RASS-3/SAS 3 or more alert. The patient is then screened for the presence of safety criteria to undergo a spontaneous breathing trial (SBT). As with the SAT, if the SBT safety screen is passed, the patient should undergo an SBT. If the patient meets any of the failure criteria, either sedation or ventilatory support is resumed and the trial begins anew the next day. Combining daily SATs with daily SBTs is associated with shorter duration of mechanical ventilation, ICU length of stay, and a 14% improvement in mortality 1 year following critical illness. (Reproduced with permission from copyright © icudelirium.org and Vanderbilt University, all rights reserved.)
Although most sedation trials have examined either targeted light sedation or daily interruption of sedatives, one recent randomized trial attempted to determine whether combining the two strategies yielded improved outcomes over using only targeted light sedation.35 Patients randomized to targeted light sedation with daily interruption had similar outcomes to those managed without targeted light sedation alone; however, it is unclear whether daily interruption of sedatives was successfully carried out in this trial. Unlike prior studies in which daily interruption of sedatives significantly reduced overall exposure to sedating medications, this approach actually increased the doses of benzodiazepines given to patients. Although this trial does not rule out the possibility that daily interruption of sedatives is beneficial when added to targeted light sedation in a way that lowers sedative doses, the study did strongly suggest that the benefit of daily interruption of sedatives is mediated through a reduction in sedative exposure. If overall exposure is not reduced, patients are less likely to benefit.
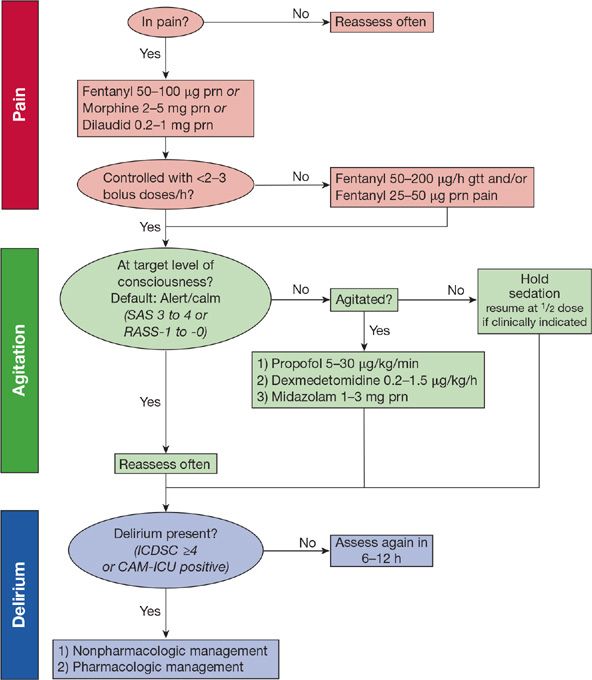
In summary, modern sedation practices (1) place an emphasis on targeted light sedation, as assessed using a validated tool for determining level of consciousness; (2) prioritize treating pain first using intravenous opiates and then, if the patient requires additional sedation, intermittent and sometimes continuous doses of a nonbenzodiazepine sedative; and (3) routinely incorporate daily interruption of drugs, maintaining a “light” level of sedation (Fig. 150-2).
Figure 150-2 Example pain, agitation, and delirium protocol. In this example protocol, pain is first assessed and, if present, IV analgesia is given first with intermittent dosing. If more than three boluses are needed in an hour, an infusion of fentanyl is started. The patient’s level of consciousness is monitored using the Richmond Agitation–Sedation Scale (RASS) or the Sedation-Agitation Scale (SAS), with a default of alert/calm or drowsy (RASS-0 to -1 or a SAS 4). If the patient is agitated despite adequate pain control, an infusion of either propofol or dexmedetomidine is preferentially used. Midazolam is begun only if the patient is withdrawing from alcohol, has a propofol intolerance or has received propofol for >96 hours. If the patient is more deeply sedated than RASS-1 or SAS 3, sedatives and analgesics are held until the patient is again alert and calm. If needed, analgesics and sedatives are resumed at half (or less) of the previous infusing dose. Each day patients undergo coordinated spontaneous awakening and breathing trials (SAT+SBT). Finally, patients are screened for delirium using either the Intensive Care Delirium Screening Checklist (ICDSC) or the Confusion Assessment Method for the ICU (CAM-ICU) and if delirium is present a cause is first sought with nonpharmacologic and pharmacologic interventions begun if no reversible cause is identified. (Reproduced with permission from copyright © icudelirium.org and Vanderbilt University, all rights reserved.)
DELIRIUM IN THE CRITICALLY ILL
Delirium during critical illness represents an acute form of brain dysfunction. Because it is frequently observed in the ICU, this syndrome was previously considered by many to be an expected, inconsequential complication of critical illness, often referred to as “ICU syndrome.”36 Multiple studies have shown, however, that delirium is associated with numerous adverse short- and long-term outcomes. Yet, it often goes unrecognized, in part because the most common presentation is that of hypoactive (quiet) delirium. This section reviews the definition of delirium, key features and differences from other forms of altered mental status, the current understanding of delirium’s pathophysiologic basis, risk factors for development, bedside tools specifically designed to assess delirium in the ICU, the short- and long-term consequences associated with delirium, and methods for preventing and treating delirium.
 DEFINITION OF DELIRIUM
DEFINITION OF DELIRIUM
The American Psychiatric Association (APA) recently updated their Diagnostic and Statistical Manual of Mental Disorders (DSM).37 The fifth edition (DSM-5) retained the core diagnostic criteria for delirium while making some changes: What was previously described as a “disturbance of consciousness” is now a “disturbance in attention,” and the manual now explicitly states that delirium cannot be diagnosed in the setting of a “severely reduced level of arousal, such as coma.” The diagnostic criteria37 for delirium are (1) a disturbance in attention (i.e., reduced ability to direct, focus, sustain, and shift attention) and awareness (reduced orientation to the environment); (2) development of the disturbance over a short period of time (usually hours to a few days), in which the development represents a change from baseline in attention and awareness and which tends to fluctuate in severity during the course of a day; (3) an additional disturbance in cognition (e.g., memory deficit, disorientation, language, visual–spatial ability, or perception); (4) the disturbances in criteria (1) and (2) are not better explained by another pre-existing, established, or evolving neurocognitive disorder and do not occur in the context of a severely reduced level of arousal, such as coma; (5) there is evidence from the history, physical examination, or laboratory findings that the disturbance is a direct physiologic consequence of another medical condition, substance intoxication or withdrawal (i.e., due to a drug of abuse or to a medication), exposure to a toxin, or is due to multiple etiologies.
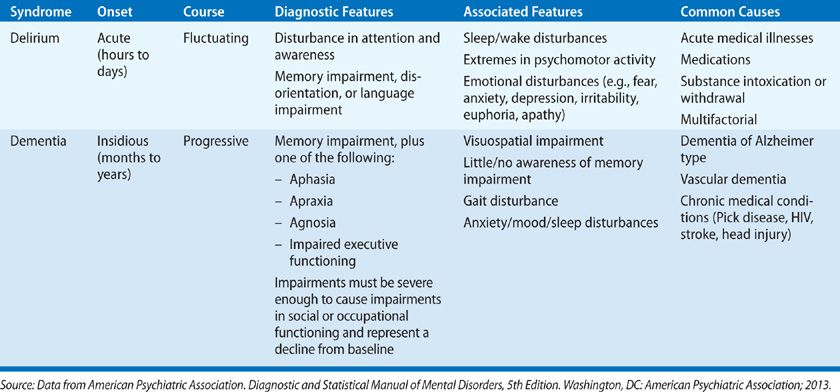
Many signs and symptoms commonly observed in delirium, such as disturbance in the sleep–wake cycle, psychomotor agitation or decreased psychomotor activity, hallucinations, delusions and perceptual disturbances, support the diagnosis, but they are neither sufficient nor required to make the diagnosis. Because these symptoms may be caused by other neurocognitive disorders, delirium is sometimes mistaken for other disorders, particularly dementia. Table 150-5 summarizes differences between delirium and dementia.
Historically, a large number of terms have been used to describe delirium in ICU patients, including encephalopathy, ICU psychosis, ICU syndrome, toxic confusional state, brain failure, subacute befuddlement, postoperative psychosis, and septic encephalopathy.38 Use of these terms can lead to misunderstanding by practitioners, and certain terms, including ICU syndrome and ICU psychosis, can imply that the syndrome is an expected part of an ICU stay.36,38 Therefore, regardless of the underlying etiology, the standardized term delirium should be used to describe a patient who meets the DSM criteria for delirium, with that underlying etiology then used to describe the delirium (e.g., use the term “delirium due to sepsis” rather than, “septic encephalopathy”).36,38
 PREVALENCE AND SUBTYPES OF DELIRIUM
PREVALENCE AND SUBTYPES OF DELIRIUM
Delirium is highly prevalent among mechanically ventilated patients in ICUs (60%–80% develop delirium at some point during the ICU stay)15,24,25,39–41 and also commonly affects nonventilated patients in the ICU setting (20%–40%).42–45 Delirium is often classified into subtypes according to the motor activity of the patient; these subtypes include hyperactive, hypoactive, and mixed delirium. Patients with hyperactive delirium are agitated, restless, and may show signs of hallucinations or delusions. Those with the hypoactive subtype are calm, lethargic, and may have decreased spontaneous movements. Finally, the mixed delirium subtype presents with features of both hyperactive and hypoactive delirium. The most common subtypes observed in the ICU are mixed and hypoactive, with purely hyperactive delirium comprising only about 1% of cases of delirium.46–48 Research is needed to determine the clinical outcomes, especially long-term, associated with specific subtypes of delirium in the critically ill. Nonetheless, as data continue to accumulate that the length of time a patient is delirious is associated with worse clinical outcomes, the fact that a majority of delirious patients in the ICU have mixed or hypoactive subtypes is an important point for clinicians, as the presence of delirium is frequently overlooked.49
 RISK FACTORS FOR DELIRIUM
RISK FACTORS FOR DELIRIUM
Delirium during critical illness is typically a multifactorial syndrome that develops when a vulnerable patient encounters a sufficiently strong insult or insults to precipitate onset of the syndrome.50 Whereas a patient with high baseline vulnerability, such as an elderly patient with underlying dementia, may require only a mild insult, such as pneumonia, a younger patient without pre-existing cognitive impairment, that is, low baseline vulnerability, may require multiple strong insults, such as severe sepsis, sedation with benzodiazepines, and immobilization. This scenario is not uncommon, given that critically ill patients are exposed on average to 11 risk factors for delirium.51
Risk factors for delirium can be thought of as predisposing factors and include baseline patient characteristics and premorbid conditions, as well as precipitating factors, such as those related to critical illness and treatment in an ICU. Baseline risk factors include older age, baseline cognitive impairment or dementia, use of alcohol or tobacco, hypertension, visual or hearing impairment, and APOE4 genotype.40,52–56 A number of precipitating factors are linked to development of delirium during critical illness, including high severity of illness, admission to an ICU for a medical illness, need for mechanical ventilation, high number of infusing medications, hypotension, acidosis, fevers, sepsis, electrolyte abnormalities, liver function test abnormalities, and anemia.41,52–55,57,58 Whereas each of these risk factors results from a patient’s critical illness, factors associated with treatment may also increase risk. Precipitating risk factors associated with treatment in an ICU include immobility, presence of gastric tubes, bladder catheters, arterial lines, sedative and analgesic medications, particularly benzodiazepines, and those factors related to the overall ICU environment, including lack of daylight, isolation, lack of visitors, and sleep disturbances.57,59
 PATHOPHYSIOLOGY OF DELIRIUM
PATHOPHYSIOLOGY OF DELIRIUM
Delirium pathophysiology, although incompletely understood, likely represents a final common pathway from many different and, perhaps, interrelated pathophysiologic mechanisms, including neurotransmitter imbalances, inflammatory responses, aberrant stress responses, and impaired cerebral perfusion. It is thought that imbalances in the neurotransmitters that maintain arousal and sleep–wake cycles – acetylcholine and dopamine – are primary drivers of delirium.60,61 Impaired central cholinergic functioning can result from derangements occurring anywhere along the pathway from acetylcholine production to its release and action of postsynaptic receptors. Acetylcholine deficiency or blockade has been associated with delirium, as has dopamine excess, likely through dopamine receptor regulation of acetylcholine activity.61–63 Other neurotransmitters, such as gamma-aminobutyric acid (GABA), norepinephrine, serotonin, glutamate, and melatonin have also been hypothesized to play a role in delirium, although the contribution of these neurotransmitters in the development of delirium is less well understood.60–65
Alterations in precursor molecules to neurotransmitters, such as dopamine, norepinephrine, serotonin, and melatonin – in particular, the large neutral amino acids (LNAAs) – have also been hypothesized to be involved in the pathogenesis of delirium.65 LNAAs, which include leucine, valine, tryptophan, tyrosine, and phenylalanine, compete for transfer across the blood–brain barrier via a single transport receptor, such that increased transport of one LNAA results in decreased transport of others. Thus, the concentration of neurotransmitters in the CNS is related to changes in serum concentrations of LNAAs, and changes in these concentrations have been associated with delirium during critical illness.66–68
Systemic inflammation, which is very common during critical illness, is also thought to play a role in development of delirium. Inflammatory molecules, such as interleukin-1β, interleukin-6, interleukin-8, tumor necrosis factor-α, prostaglandins, and blood-borne molecules, such as lipopolysaccharide, are released in response to infection, tissue damage, and surgery. These molecules interact with the brain via direct autonomic neural pathways, second-messenger systems, active transport across the blood–brain barrier, and through disruptions in the blood–brain barrier, resulting in neuroinflammation.69–71 Neuroinflammation triggers CNS production of additional inflammatory cytokines and metalloproteinases, release of reactive oxygen species, and expansion and activation of microglia, resulting in alterations in CNS perfusion, impaired neurotransmitter production, and derangements in oxidative metabolism, ultimately leading to neuronal damage and cell death.69,71 Neuronal death results in a functional disconnect between neuroanatomical structures, culminating in the neurobehavioral changes observed in delirium.69
 SCREENING TOOLS FOR DELIRIUM
SCREENING TOOLS FOR DELIRIUM
Since many delirious patients are lethargic or calm, rather than agitated, delirium in the ICU is overlooked nearly 70% of the time.49 Thus, current expert guidelines recommend that all patients in an ICU be routinely monitored using a well-validated delirium-screening tool.5 Two screening tools that were developed specifically for assessment of critically ill adult patients, the Confusion Assessment Method for the ICU (CAM-ICU) and the Intensive Care Delirium Screening Checklist (ICDSC), have been the most rigorously validated and widely implemented and, thus, are recommended in expert guidelines.5 Validated before the recent release of DSM-537 criteria, both the CAM-ICU and the ICDSC were compared in validation studies with expert psychiatric examinations applying the DSM-IV72 criteria for delirium and were found to have high sensitivity and specificity, as well as excellent interrater reliability when performed by ICU physicians and nurses.39,44,73–77 Both screening tools identify features of delirium that remain part of the DSM-5 definition, such that the tools are considered current and valid in the DSM-5 era.
When using either the CAM-ICU or the ICDSC, the first step in assessing for delirium is to assess the patient’s level of arousal/consciousness (see Assessing Agitation and Level of Consciousness in the Critically Ill); as stated in the DSM-5, delirium cannot be assessed in the setting of coma. In validation studies of the CAM-ICU, this was operationalized by determining that patients were arousable to voice (e.g., RASS-3 or more awake) before proceeding with the CAM-ICU assessment. Similarly, the ICDSC excludes patients in coma or stupor from delirium assessment. Once level of consciousness is assessed, the second step in the delirium screen is to assess the content of consciousness using either the ICDSC or the CAM-ICU.
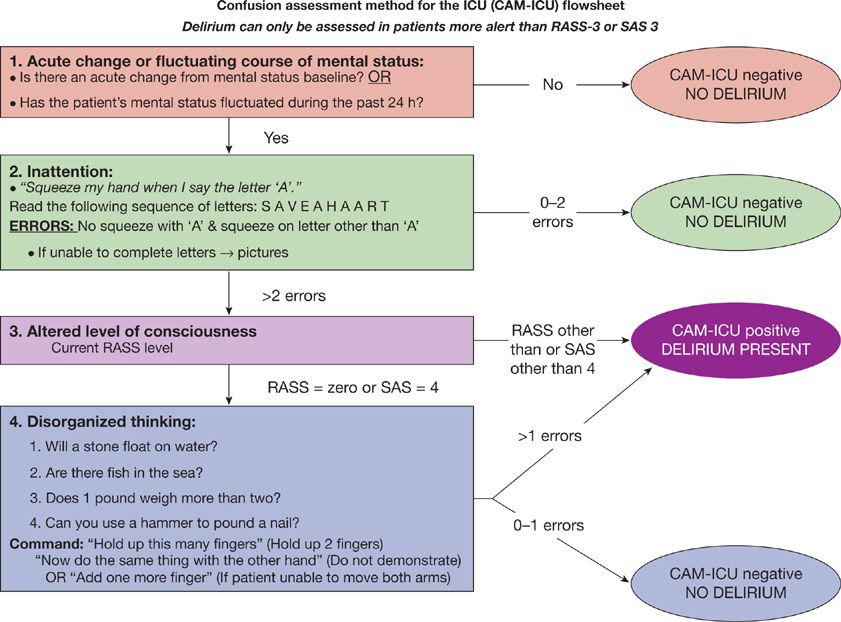
The CAM-ICU was developed based on the full Confusion Assessment Method78 but tailored to the specific needs of patients in the ICU (e.g., it can be performed in both nonintubated and intubated patients, even in the setting of sedation [as long as the patient is not comatose]). During what is typically a 1- to 2-minute evaluation, the CAM-ICU assesses four key features of delirium: (1) Acute change or fluctuation in mental status, (2) inattention, (3) altered level of consciousness, and (4) disorganized thinking.39,74 The CAM-ICU is positive, that is, the patient is considered to have delirium, if features 1 and 2 are present and at least feature 3 or 4 is present. See Figure 150-3 for instructions on how to assess each feature in the CAM-ICU. This tool has been translated into over 20 different languages, and many of these translated versions have been validated.
Figure 150-3 The Confusion Assessment Method for the ICU (CAM-ICU). The CAM-ICU assesses for the four features of delirium. Feature 1 is an acute change in mental status or a fluctuating mental status (orange box), feature 2, is inattention, (green box), feature 3, is altered level of consciousness (purple box), and feature 4, is disorganized thinking (blue box). A patient screens positive for delirium if features 1 and 2 and either feature 3 or feature 4 are present. (Reproduced with permission from copyright © 2002, E. Wesley Ely, MD and Vanderbilt University.)
The ICDSC is an 8-item checklist of delirium symptoms assessed over an 8- to 24-hour period. Providers monitor patients for the symptoms on the checklist during that period of time (e.g., during a nursing shift) and mark items on the checklist as they are observed. The symptoms include altered level of consciousness, inattention, disorientation, hallucinations/delusions/psychosis, psychomotor agitation or retardation, inappropriate speech or mood, sleep/wake disturbances, and fluctuation of symptoms. One point is given for each symptom observed; a score of four or greater indicates delirium (Table 150-6).