Chapter 23 Diagnosis and Surgical Management of the Visceral Ischemic Syndromes
Although the causal relationship between acute mesenteric vascular occlusion and intestinal gangrene had been known for centuries, it was not until 1895 that the first successful case of preoperative recognition and treatment by intestinal resection was reported by Elliot.1 Recognition of the chronic form soon followed, with the term angina abdominis applied by Goodman to illustrate the similarities to the newly described angina pectoris.2 Indisputable evidence was provided in 1936 by Dunphy,3 a surgical resident at the Peter Bent Brigham Hospital in Boston who described the clinical course of a 47-year-old man with weight loss and periumbilical pain out of proportion to the findings on physical examination. The patient died suddenly in the hospital, and postmortem examination revealed chronic mesenteric disease with fresh thrombus completely occluding the celiac trunk. Dunphy reviewed 12 other deaths from mesenteric vascular occlusion and found an antecedent history of chronic recurrent abdominal pain in 7.
The first successful reports of mesenteric revascularization appeared in 1958.4 In that year, Shaw and Maynard4 from the Massachusetts General Hospital reported two cases of superior mesenteric artery (SMA) thrombosis superimposed on atherosclerotic occlusive disease. Both patients were treated with SMA thromboendarterectomy and survived. The surgeons made the astute observation that although the responsible atherosclerotic lesions involved all three mesenteric arteries, they were confined to the proximal segments, such that vascular reconstruction was technically feasible.
Vascular Anatomy
The mesenteric circulation consists primarily of three branches of the abdominal aorta (Figure 23-1): the celiac axis, the SMA, and the inferior mesenteric artery (IMA). Their multiple branch points and interconnections form such a rich anastomotic network that compromise of two of the three major arteries is usually required for the development of chronic ischemic symptoms. Knowledge of the normal and variant anatomy is essential for surgical diagnosis and revascularization.
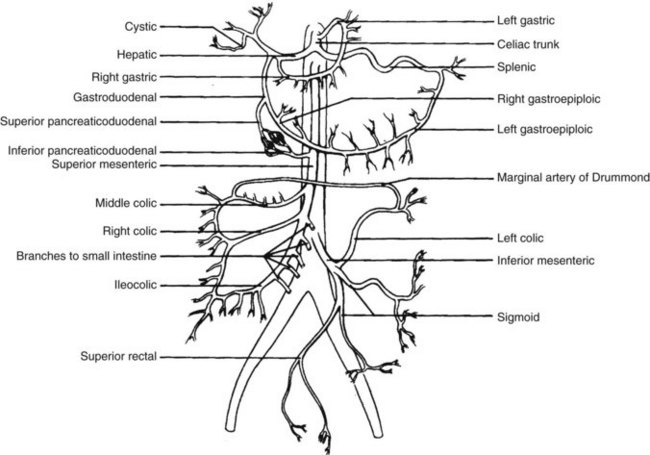
FIGURE 23-1 The mesenteric circulation.
(From Schwartz LB, Davis RD Jr, Heinle JS, et al: The vascular system. In Lyerly HK, Gaynor JW Jr, editors: The handbook of surgical intensive care, ed 3, St Louis, 1992, Mosby Year Book, p 287.)
Acute Ischemia
Pathophysiology
There are four primary causes of acute mesenteric ischemia (AMI): embolization to the SMA (approximately 50% of all cases), thrombosis of a preexisting atherosclerotic lesion at the origin of the vessel (20%), nonocclusive mesenteric ischemia (20%), and mesenteric venous thrombosis (10%).5,6 In earlier series, the phenomenon of nonocclusive mesenteric ischemia was not well appreciated and was frequently misdiagnosed as acute venous occlusion. For example, Cokkinis7 reported in 1935 that acute mesenteric venous thrombosis accounted for the majority of cases of AMI.
Other unusual arteriopathies, such as Takayasus arteritis, fibromuscular dysplasia, and polyarteritis nodosa, may first present with intestinal ischemia. Isolated dissections of the SMA also have been reported,8 although the more common mechanism is extension of dissections of the descending thoracic aorta into the SMA and celiac axis.9,10
If untreated, intestinal ischemia commonly leads to intestinal infarction. Tissue loss may result from both hypoxia during flow interruption and reperfusion injury once intestinal arterial blood flow is restored. Reperfusion injury is principally mediated by activation of the enzyme xanthine oxidase and the recruitment and activation of circulating polymorphonuclear neutrophils (PMNs).11,12 In the presence of oxygen and hypoxanthine (a by-product of adenosine triphosphate metabolism), xanthine oxidase produces oxygen-derived free radicals that cause severe local tissue injury through lipid peroxidation, membrane disruption, and increased microvascular permeability.13 PMNs are attracted to reperfused tissue by the local secretion of cytokines (tumor necrosis factor-α, interleukin-1, platelet-derived growth factor) by ischemic endothelium.14–16 Subsequent rolling, adherence, and activation of the PMNs in the microcirculation result in the secretion of myeloperoxidase, collagenases, and elastases that can further injure the already ischemic and vulnerable tissue.17–19 Activation of the inflammatory cascade may also have systemic effects, with cardiac, pulmonary, and other organ system dysfunction.20
Acute Mesenteric Arterial Embolism
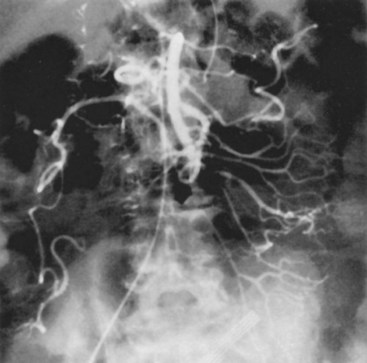
Most mesenteric arterial emboli originate from left atrial or ventricular mural thrombi or cardiac valvular lesions. These thrombi are usually associated with cardiac dysrhythmias such as atrial fibrillation or hypokinetic regions from previous myocardial infarctions. The majority of mesenteric emboli lodge in the SMA because of its high basal flow and nearly parallel course to the abdominal aorta. Only 15% of SMA emboli remain impacted at the origin of the vessel. The majority of emboli progress distally 3 to 10 cm to the tapered segment of the SMA just past the origin of the middle colic artery (Figure 23-2). A substantial fraction (10% to 15%) of mesenteric emboli are associated with concurrent emboli to another arterial bed.21 Intestinal ischemia owing to embolic arterial occlusion can be compounded by reactive mesenteric vasoconstriction, which further reduces collateral flow and aggravates the ischemic insult.
Acute Mesenteric Arterial Thrombosis
As noted previously, an unusual cause of AMI is aortic dissection involving the origins of the visceral vessels. The intimal flap of the dissection can exclude, compress, or extend into the visceral vessels, resulting in acute thrombosis. The symptoms of bowel ischemia may be masked by the pain associated with the aortic dissection, leading to a delay in diagnosis and treatment. AMI following coronary artery bypass grafts is rare but highly lethal, with mortality rates as high as 70%. As would be expected, ischemia occurs in patients with severely stenotic mesenteric vessels that occlude during the nonpulsatile perfusion of extracorporeal bypass.22,23
Nonocclusive Mesenteric Ischemia
Mesenteric ischemia unassociated with anatomic arterial or venous obstruction can occur during periods of low cardiac output. Such low-flow states can result from cardiac failure, sepsis, or administration of α-adrenergic agents or digitalis compounds. Although less common, mesenteric vasospasm can also follow elective revascularization procedures for chronic SMA occlusion, in which case vasoconstriction of small- and medium-size vessels is precipitated by early enteral feeding.24 The older mean age of the population has produced a group of people with severe medical problems at risk for this type of mesenteric ischemia. The diagnosis is made at the time of angiography. Radiographic criteria suggesting the diagnosis include (1) narrowing of the origins of multiple branches of the SMA; (2) alternate dilatation and narrowing of the intestinal branches (the “string-of-sausages” sign); (3) spasm of the mesenteric arcades; and (4) impaired filling of the intramural vessels.25 The mortality of this specific subset of patients is relatively high regardless of treatment, owing to the serious underlying medical conditions and the frequent delay in diagnosis.26
Mesenteric Venous Thrombosis
Mesenteric venous thrombosis (MVT) is thrombosis of the veins draining the intestine (inferior mesenteric, superior mesenteric, splenic, and portal veins). The obstruction in venous return leads to edema, distension, and eventual infarction of affected segments. Primary MVT is idiopathic, although given the improved understanding of predisposing conditions, the number of patients in this category is diminishing. Patients in whom a causative factor is identified are said to have secondary MVT. These factors include myriad clinical syndromes, including trauma, surgery, cancer, cirrhosis, pancreatitis, dehydration, and increasingly recognized hypercoagulable syndromes such as polycythemia vera, thrombocytosis, protein C and S deficiency, antithrombin III deficiency, antiphospholipid antibody syndrome, and factor V Leiden mutation (activated protein C resistance).27
Patients with MVT have a somewhat different presentation from those who have ischemia caused by arterial obstruction; the onset of symptoms may be insidious and the findings more subtle. Pain out of proportion to the physical examination is still an essential feature. The test of choice to confirm the diagnosis is contrast-enhanced computed tomography (CT), although duplex scanning and magnetic resonance imaging (MRI) are gaining popularity. Thrombus is located in the superior mesenteric vein in 70% of patients, with portal and inferior mesenteric vein thrombus found in about 30%.28
Symptomatic acute MVT is a lethal disease, with a 30-day mortality of about 25% and a 3-year survival of 35%.28 Patients with evidence of chronic thrombosis fare somewhat better, because collateral venous channels form to augment intestinal venous drainage.
Clinical Presentation and Diagnosis
Ancillary laboratory evaluations often reveal an increase in hemoglobin and hematocrit, consistent with hemoconcentration. There is a marked leukocytosis with a predominance of immature white blood cells (left shift). Although no specific laboratory findings are diagnostic, serum levels of amylase, lactic dehydrogenase, creatine phosphokinase, and alkaline phosphatase, singly or severally, are often elevated, along with a metabolic acidosis with a persistent base deficit.29 Unfortunately, most of these abnormalities do not develop until after bowel necrosis has occurred.
Plain abdominal radiographs are used to exclude other potential causes of abdominal pain rather than confirm the diagnosis of AMI. In fact, completely normal plain abdominal films are seen in more than 25% of patients with mesenteric ischemia.30 Subtle signs of AMI on plain abdominal films include a dynamic ileus and distended air-filled loops of bowel. Bowel wall thickening from submucosal edema or hemorrhage can be prominent, especially in cases of acute MVT. In advanced stages, pneumatosis of the bowel wall and portal vein gas portend an extremely poor prognosis.
Duplex ultrasonography may be of some benefit in visualizing flow in the SMA and celiac axis. With expert technical assistance, these tests can document proximal stenoses in the SMA or celiac axis or complete occlusion of these vessels.31 In newer series, color Doppler ultrasonography has been shown to be a valuable screening tool for AMI; it is far more specific than clinical evaluation alone.32,33 Unfortunately, a significant percentage of patients at risk for mesenteric ischemia have dilated air-filled loops of bowel that make ultrasonography difficult if not impossible.
CT of the abdomen and pelvis can delineate subtle changes consistent with subacute bowel ischemia such as focal or segmental bowel wall thickening.34 Thrombus within the mesenteric veins or the lack of opacification of the veins after intravenous contrast administration is often seen in MVT.35 Nonenhancement of the arterial vasculature with timed intravenous contrast injections can be noted in acute mesenteric arterial thrombosis or embolization. CT scans can also vividly demonstrate pneumatosis or portal vein gas.
Advances in contrast-enhanced and cine phase magnetic resonance angiography have allowed better visualization of the visceral vasculature and have a more important role in the diagnosis of AMI.36 Specifically, when MRI is coupled with magnetic resonance oximetry, both anatomic and physiologic information regarding the mesenteric circulation can be obtained.37
Treatment Options
Contrast-enhanced imaging studies, such as CT angiography, should be performed immediately. Although mesenteric angiography remains a definitive diagnostic tool, it is increasingly used in association with planned endovascular treatment. In select patients with an early diagnosis of SMA embolus unassociated with bowel necrosis, a trial of thrombolytic therapy and angioplasty may be appropriate.38,39 Such treatments are usually limited to patients with abdominal pain for less than 8 hours’ duration without signs of peritoneal irritation. If lysis is not evident within 4 hours of commencing high-dose thrombolytic therapy, or if peritoneal signs develop, the infusion should be discontinued and immediate surgical exploration should be performed.
An increasing number of case reports and series have detailed the use of percutaneous angioplasty to dilate significant atherosclerotic plaques of the SMA that are unmasked by thrombolytic therapy.40–42 Owing to the variable angle of the origin of the SMA from the aorta, placement and removal of the angioplasty catheter and stent placement may be more difficult than for lower extremity angioplasties.10 Restenosis rates range from 25% to 50% in earlier series,40,41 but the largest single-center retrospective review to date demonstrated more promising results. In a 9-year series of 70 patients with AMI, the Cleveland Clinic vascular group achieved an 87% success rate in endovascular treatment of AMI. Patients in the endovascular group were slightly older (65 ± 12 vs. 60 ± 13 years), were weighted with more thrombotic occlusion cases (72% vs. 64%), and had longer duration of symptoms (median, 62 vs. 26 hours). The primary mode of endovascular treatment was thrombolysis infusion; additional therapy included vasodilator (papaverine or nitroglycerin) therapy (51%), mechanical thrombectomy (12%), and PTA plus stent (33%). Successful endovascular therapy resulted in fewer laparotomies (69% vs. 100% in traditional therapy), lower rates of acute renal failure (27% vs. 50%) and pulmonary failure (27% vs. 64%), and lower mortality rates (36% vs. 50%).43 Those who failed endovascular treatment had the same high 50% mortality rate as the open surgical group. Still, while endovascular therapy is increasingly used for AMI at major center, open surgical revascularization remains the predominant treatment across the United States.44
When an aortic dissection involves the origin of one or more of the visceral vessels, endovascular repairs have been attempted through stent placement45,46 and balloon fenestration of the dissection septum.47 Although such minimally invasive treatments are highly attractive, they are currently limited by the frequently rapid onset of ischemic symptoms, inability to gain access to the dissection channel, or the need for surgical repair of an associated aortic aneurysm.
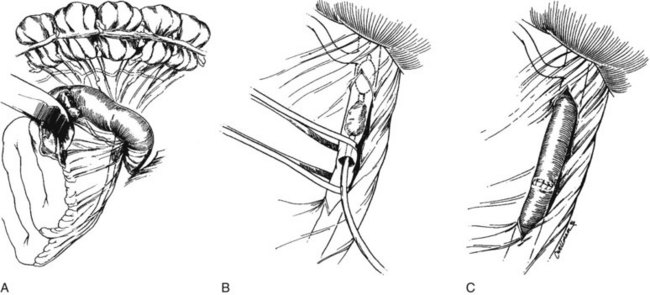
To achieve adequate exposure, the transverse colon is retracted superiorly, and the fourth portion of the duodenum is mobilized to the ligament of Treitz. The SMA is identified by palpation of the root of the mesentery. If the cause of mesenteric ischemia is an embolus, a more proximal SMA pulse is often noted. The SMA is encircled at or just distal to the level of obstruction (Figure 23-3A), and a transverse arteriotomy is made. Balloon-tipped embolectomy catheters are inserted retrograde, and the embolus is extracted (see Figure 23-3B). Embolectomy catheters should also be passed distally to ensure that no fragmentation of the clot or discontinuous thrombosis has occurred. Transverse arteriotomies are closed primarily with interrupted fine monofilament sutures to ensure that the vessel is not stenosed (see Figure 23-3C). If a longitudinal arteriotomy is required, closure is best accomplished with a vein patch. Appropriately selected longitudinal arteriotomies can also be used for distal anastomoses of bypass grafts if thrombectomies are unsuccessful in obtaining arterial inflow.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree