Twenty-five to 35 percentage of stroke cases are cryptogenic, and it has been demonstrated that paroxysmal atrial fibrillation (AF) is the causal agent in up to 25% of these incidents. The purpose of this study was to investigate if left atrial (LA) parameters have value for diagnosing paroxysmal AF in patients with ischemic stroke (IS) and transient ischemic attack (TIA). We retrospectively analyzed 219 patients who after acute IS or TIA underwent a transthoracic echocardiographic examination. Patients were designated as patients with paroxysmal AF if they had one or more reported incidents of AF before or after their echocardiographic examination. Patients in the paroxysmal AF group were significantly older and had higher CHA 2 DS 2 -VASc score than patients without paroxysmal AF (p <0.05 for both). None of the conventional echocardiographic parameters were significantly associated with paroxysmal AF. However, the atrial measurements evaluating LA function (min LA volume and LA emptying fraction) were significantly different (LA emptying fraction: 45% ± 10% vs 50% ± 10%, p = 0.004; minimal LA volume: 30.2 ml ± 17.3 ml vs 24 ml ± 10 ml, p = 0.035 in patients with paroxysmal AF, even after adjustment for age, gender, CHA 2 DS 2 -VASc score, and stroke severity [p <0.05 for both]). By combining the cut-off values of age, LA emptying fraction, and minimal LA volume the diagnostic accuracy of paroxysmal AF was improved, resulting in a sensitivity of 95% and negative predictive value of 97%. In conclusion, in patients with IS and TIA, LA function measurements (minimal LA volume and LA emptying fraction) are independently associated with paroxysmal AF and may improve risk stratification for paroxysmal AF presence after IS or TIA.
Studies have shown that echocardiographic measurements of left atrium volume (LAV) and function could be early predictors of ischemic stroke (IS), transient ischemic attack (TIA), and atrial fibrillation (AF). Therefore, measurements of LAV and function may improve the diagnosis of AF after IS and TIA, as almost 80% of all patients admitted with IS or TIA will have an echocardiographic examination performed in the diagnostic workup, and an assessment of the LAV and LA function would be easy to implement. Therefore, the aim of the present study was to evaluate if measurements of LAV and LA function could improve the identification of AF in patients experiencing IS or TIA.
Methods
This retrospectively planned and conducted analysis was based on patients from 2 clinical registries in the Department of Neurology, Bispebjerg Hospital. The 2 cohorts (A and B) of patients with acute cerebrovascular ischemia were admitted in the period from April 2009 to March 2012 (cohort A) and from February 2010 to September 2012 (cohort B). Cohort A consisted of consecutive patients (n = 848) admitted within 4.5 hours of stroke onset to a single center and discharged with a diagnosis of TIA or IS in whom computed tomography angiography was performed on admission; the population has previously been described. Cohort B consisted of consecutive patients (n = 737) discharged from a single center with a final diagnosis of TIA and magnetic resonance imaging performed after symptom onset. Echocardiography was not part of standard workup and was only performed at the discretion of the treating neurologist if the examination was found indicated based on clinical findings. Echocardiographic findings were not included in any of the 2 registries, and data were retrieved and reviewed as part of the present analysis. Telemetry (24 hours) was part of standard monitoring together with electrocardiography in 12 leads on admission.
A diagnosis of AF was made by review of electronic medical files and supplementary notes from the echocardiographic examination. The electronic medical files include reports and diagnostic codes from patient hospitalizations and out-patient visits. Patients were considered as having AF when at least one reported incident of paroxysmal AF was found, this included both incidents before and after the echocardiographic examination. The clinical baseline data and medical history were obtained by the same procedure.
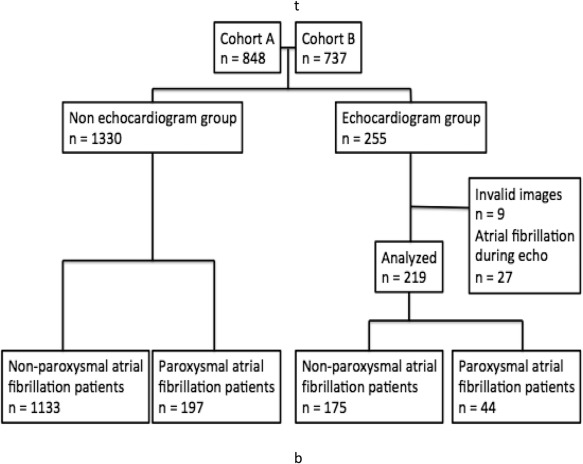
The population (n = 1,585) was dichotomized according to existence of an echocardiographic examination, 255 were included into the analysis. Further 9 patients were excluded because of invalid echocardiographic images and 27 because AF was observed during the examination. Figure 1 shows the patient flow. The registry on which the analysis was based was approved by the Danish Data Protection Agency file no 2009-41-3824.
All patients had a conventional 2-dimensional transthoracic echocardiography (Phillips iE 33, Netherlands) performed, and the analyses of the echocardiography were done offline with the commercially available software–Xcelera quantification software. Only patients in sinus rhythm during the echocardiographic examination were included. A single investigator who was blinded to all other information did all the echocardiographic analysis.
The left ventricle (LV) end-diastolic measurements (interventricular septal wall thickness in diastole, LV internal diameter in diastole, and LV posterior wall thickness in diastole) were obtained through the parasternal long-axis view at the tip of the mitral valve leaflets. The LA dimensions (maximal and minimal LA diameter) were acquired in LV end-systole and -diastole. The LA fractional shortening was calculated by dividing the difference between maximal and minimal LA diameter with maximal LA diameter.
The LV volumes and lengths in end-diastole and -systole were obtained by the modified biplane Simpson’s method. The LAVs in end-diastole and -systole were obtained by the same method. The LA emptying fraction was calculated by dividing the difference between maximal and minimal LAV with maximal LAV.
Pulsed wave Doppler at the apical 4-chamber view was used to record mitral inflow at the tips of the mitral leaflets. Peak velocity of early diastolic filling of the ventricle (E), peak velocity of atrial diastolic filling of the ventricle (A), and deceleration time of the early filling were measured and the E/A ratio was calculated. Pulsed wave tissue Doppler imaging was used to obtain the peak longitudinal early diastolic velocity (e’) and the peak longitudinal late diastolic velocity (a’) at the lateral and septal mitral annulus, respectively. e’ was used to calculate E/e’.
Using m-mode at the 4-chamber apical view, the right ventricle tricuspid annular plane systolic excursion was measured.
In Tables 1 to 4 , proportions were compared using chi-square test, continuous Gaussian distributed variables with Student’s t test. All continuous variables are expressed as mean values ± standard deviation, and categorical variables as frequencies (percentages). Odds ratios were calculated by logistic regression analyses. Receiver operating characteristics (ROC) curves were constructed for age, E/e’, CHA 2 DS 2 -VASc score, maximal LAV, minimal LAV, LV ejection fraction, and LA emptying fraction, and area under the curve (AUC) was found. Age, LA emptying fraction and minimal LAV were included as predictors in separate logistic regressions and in the same logistic regression model. We thereby combined the information gained from each of the variables to give the best diagnostic accuracy. Improvement in diagnostic utility was tested by calculating AUC of the ROC curves for the variables individually and for the 3 variables included in the same model. The difference between the AUC and the ROC curves were performed using nested logistic regression models and tested with the roccomp function. From the ROC curves constructed for each of the variables (LA emptying fraction, age, and minimal LAV), the optimal cut-off values were found with the highest sensitivity and specificity. SPSS for Mac version 20.0 and STATA Statistics/Data analysis, SE 12.0 (StataCorp, Texas) were used for statistical calculations.
| Variable | Paroxysmal Atrial Fibrillation | ||
|---|---|---|---|
| No n = 175 | Yes n = 44 | P-value | |
| Age (years) | 51.5±13.5 | 61.5±12.9 | <0.001 |
| Men | 60% | 50% | 0.23 |
| Diabetes mellitus | 11% | 5% | 0.21 |
| Hypertension | 42% | 43% | 0.88 |
| Hypercholesterolemia | 67% | 64% | 0.75 |
| National Institutes of Health Stroke Scale | 1.5±3.3 | 2.9±4.9 | 0.07 |
| CHA 2 DS 2 -VASc | |||
| Mean | 3.3±1.3 (n=166) | 3.8±1.3 (n=44) | 0.042 |
| 2 | 84% (n=46) | 16% (n=9) | 0.005 |
| 3 | 86% (n=63) | 14% (n=10) | |
| 4 | 74% (n=29) | 26% (n=10) | |
| 5 | 52% (n=14) | 48% (n=13) | |
| 6 | 92% (n=11) | 8% (n=1) | |
| 7 | 75% (n=3) | 25% (n=1) | |
| No echocardiogram n = 1330 | Valid sinus rhythm echocardiogram n = 219 | P-value | |
|---|---|---|---|
| Age (years) | 64.7±15.8 (n = 1146) | 53.5±14.0 (n = 219) | <0.001 |
| Men | 54% (n=609) | 58% (n=127) | 0.30 |
| Diabetes mellitus | 10% (n=112) | 10% (n=20) | 0.89 |
| Hypertension | 60% (n=680) | 42% (n=89) | <0.001 |
| Hypercholesterolemia | 52% (n=509) | 66% (n=134) | <0.001 |
| Paroxysmal atrial fibrillation | 17% (n=197) | 20% (n=44) | 0.31 |
| National Institutes of Health Stroke Scale | 4.2±7.6 (n = 1330) | 1.8±3.7 (n = 219) | <0.001 |
| Variable | Paroxysmal atrial fibrillation | ||
|---|---|---|---|
| No n = 175 | Yes n = 44 | P-value | |
| Left Ventricular Ejection Fraction (%) | 47±8 | 46±9 | 0.30 |
| Left Ventricular volume in diastole (mL) | 118±29 | 118±35 | 0.95 |
| Left Ventricular volume in systole (mL) | 63±21 | 65±24 | 0.69 |
| Left Ventricular Internal Diameter in diastole (cm) | 4.7±0,7 | 4.6±0.6 | 0.48 |
| Interventricular Septal Wall Thickness in diastole (cm) | 0.99±0.20 | 1.03±0.18 | 0.26 |
| Left Ventricular Posterior Wall Thickness in diastole (cm) | 1.03±0.66 | 0.97±0.20 | 0.53 |
| Peak velocity of early diastolic filling of the ventricle (E) (cm/s) | 73±17 ∗ | 83±27 ∗ | 0.047 |
| Peak velocity of atrial diastolic filling of the ventricle (A) (cm/s) | 68±21 † | 70±23 † | 0.69 |
| E/A-ratio | 1.1±0.36 ‡ | 1.3±0.78 ‡ | 0.18 |
| Deceleration time of the early filling (s) | 0.22±0.04 § | 0.22±0.06 § | 0.86 |
| Peak longitudinal early diastolic velocity (e’) (cm/s) | 10.9±3.6 ¶ | 10.8±3.7 ¶ | 0.92 |
| E/e’ | 7.3±2.7 || | 8.0±3.0 || | 0.22 |
| Tricuspid annular plane systolic excursion (cm) | 2.4±0.49 ∗∗ | 2.4±0.47 ∗∗ | 0.73 |
∗ Data were only available for 153 of the 175 patients without PAF and 39 of the 44 patients with PAF.
† Data were only available for 151 of the 175 patients without PAF patients and 38 of the 44 patients with PAF.
‡ Data were only available for 151 of the 175 patients without PAF and 38 of the 44 patients with PAF.
§ Data were only available for 149 of the 175 patients without PAF and 38 of the 44 patients with PAF.
¶ Data were only available for 141 of the 175 patients without PAF and 33 of the 44 patients with PAF.
‖ Data were only available for 141 of the 175 patients without PAF and 33 of the 44 patients with PAF.
∗∗ Data were only available for 145 of the 175 patients without PAF and 37 of the 44 patients with PAF.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


