Implanted devices can provide objective assessment of physical activity over prolonged periods. The purpose of this study was to investigate the prognostic value of device-measured physical activity data compared with a six-minute walk test (6MWT) in predicting clinical response to cardiac resynchronization therapy (CRT). This was a single-center study in which patients who underwent CRT for standard indications were evaluated. Daily physical activity and 6MWT were evaluated postimplant at 1, 3, and 6 months. The primary end point was a composite of heart failure hospitalization, transplant, left ventricular (LV) assist device, and all-cause death at 3 years. Echocardiographic response, defined as a ≥10% improvement in LV ejection fraction (LVEF), at 6 months was the secondary end point. About 164 patients were included: average age was 67.3 ± 12.9 years, 77% were men, baseline LVEF was 25% ± 7%. Kaplan-Meier curves showed superior freedom from the composite end point in the highest tertile of both 6MWT and physical activity compared with the lowest tertile (41 vs 23 cases, respectively, p <0.001) for 6MWT and for activity (22 vs 7 cases, respectively, p = 0.001). In an adjusted multivariate model, independent predictors of improved clinical outcome included 1-month physical activity (hazard ratio 0.546, 95% confidence interval [CI] 0.361 to 0.824, p = 0.004) and 6MWT (hazard ratio 0.581, 95% CI 0.425 to 0.795, p = 0.001). An additional hour of higher activity at 1 month translated to a 1.38 times (95% CI 1.075 to 1.753, p = 0.011) higher likelihood of improved echocardiographic response. In conclusion, device-based measures of physical activity may be useful in predicting echocardiographic reverse remodeling and long-term clinical outcome in patients receiving CRT.
For effective clinical management of patients with heart failure (HF) living with cardiac resynchronization therapy (CRT), early risk-stratification to identify potential nonresponse and also finding simple and reliable method to evaluate the functional status is important. Although six-minute walk test (6MWD) and cardiopulmonary exercise testing have been shown to provide prognostic information for all-cause hospitalization and mortality in HF population, they have significant limitations both logistically and in their reproducibility. Of note, both of these tests can only be performed periodically and are thereby reflective of the clinical status of the patient only at that particular point in time. More recently, data derived from implantable devices have gained considerable attention as risk stratifying measures. Most contemporary devices have the ability of measuring daily physical activity through sensors incorporated within the device. The activity information can thereby be acquired on a daily basis over prolonged periods. Despite the ease of acquiring this information, there are limited data examining the relationship of device-based physical activity measures and clinical response to CRT. The purpose of this study was to investigate the prognostic value of device-measured physical activity data compared with a one-time 6MWT in predicting clinical response to CRT.
Methods
This study evaluated 164 consecutive patients enrolled in the Massachusetts General Hospital Multidisciplinary CRT Clinic between April 2004 and April 2010 in which detailed device diagnostic data were available. Demographic and outcome data were collected prospectively on each patient seen in the CRT clinic. The present project and proposed analysis were approved by the Massachusetts General Hospital Institutional Review Board and Ethics Committee. In the multidisciplinary clinic, patients were seen at 1-, 3-, and 6-month postimplant by HF, echocardiography, and electrophysiology specialists during an integrated care visit. The CRT was indicated according to the approved guidelines. Data points of physical activity that were collected included 6MWT and physical activity data from the devices.
The 6MWT was conducted according to a standardized manner. The test was carried out in a straight, unimpeded 20 m long hallway, where chairs were positioned at both ends providing patients a place for rest if needed. The subjects were instructed to walk as much as possible, but they were permitted to slow down or stop as necessary. They were encouraged in a standardized manner without influencing their walking speed. Before and immediately after the test, finger pulse oximetry (SpO 2 ) was measured. If resting SpO 2 was <88%, the patient was considered not eligible to begin the test.
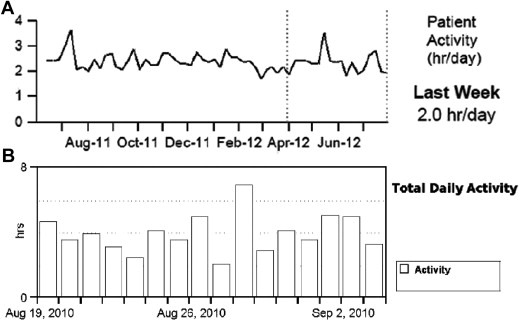
Device-based physical activity data were obtained through device interrogations during follow-up visits. Diagnostic data from devices of 2 different companies (Medtronic and St. Jude Medical) were used for this analysis: 86 patients had Medtronic and 79 patients had St. Jude Medical device implanted. Implanted devices measure activity by an accelerometer, which consists of a piezoelectric crystal and a moving component that enables the sensing of the intensity and frequency of body motion and then converts it into an electrical signal. The sensor thresholds of measurements and the processing algorithms are variable within the 2 companies. Importantly, device measures of physical activity were displayed differently between the device companies. Medtronic devices (Medtronic Inc., Minneapolis, Minnesota) recorded the physical activity regardless of mode and rate response programing. The data were averaged for each 7-day period, and the average was plotted for the latest day of that period. The trend demonstrated a line connecting each weekly average value ( Figure 1 , panel A). The activity threshold was nominally set so that a continuous 60 to 70 step per minute walk was registered as active for that minute. Most activities of daily living (such as doing dishes, vacuuming, and removing the garbage) were considered as “active.” St. Jude Medical device (St Jude Medical Inc., Sylmar, California) report displayed bar graphs that indicated the number of hours the patients are active each day ( Figure 1 , panel B). Activity was defined as input to the sensor that exceeded the resting heart rate. That threshold rate was recorded in the first 48 hours after implant. Notably, there were no duration criteria for the activity level. Importantly, both of the manufacturers reported the daily activity in unit of hour/day.
Demographic and clinical data were obtained prospectively for all the patients. Transthoracic echocardiography was performed before CRT implantation, at 1 month during device optimization and then uniformly at 6 months of postimplant. All echocardiograms were performed on commercial ultrasound machines (Philips iE33, Koninklijke Philips N.V., Eindhoven, The Netherlands; SONOS 5500/7500; Andover, Massachusetts and General Electric Vivid 7, GE Healthcare; Milwaukee, Wisconsin). Left ventricular (LV) end-diastolic and LV end-systolic dimensions were measured from the parasternal long-axis view. LV ejection fraction (LVEF) was calculated by the following standard biplane method of discs from the apical 4- and 2-chamber views. Echocardiographic variables collected were LVEF, LV internal diameter in diastole, and LV internal diameter in systole.
All patients were followed up for hard clinical end points, that is, all-cause mortality, HF hospitalizations, LV assist device implantation, and cardiac transplant. For both cohorts, HF hospitalization was defined as inpatient admission with signs and/or symptoms of HF, including shortness of breath, peripheral edema, and/or congestion on the chest radiograph, and improvement of these signs and/or symptoms with medical therapy. The clinical end points of the study evaluated were (1) HF hospitalization and (2) a composite of HF hospitalization, transplant, implantation of LV assist device, and all-cause death. Echocardiographic response was defined as a >10% improvement in LVEF, after CRT. Notably, clinical outcomes were reconfirmed with review of the electronic medical record and comparison with the social security death index.
Statistical analyses were performed by using SPSS version 21.0 and SPSS Sample Power 3.0.1 (SPSS Inc, Chicago, Illinois). Because there are no defined categories for daily activity and 6MWT, patients were divided into tertiles based on the activity results and 6MWT distances. The comparison of baseline characteristics was performed according to 1-month daily activity tertiles. Results are presented as mean ± SD or median and interquartile range for continuous variables and frequencies for categorical variables. For the assessment of differences, analysis of variance, Student t , Wilcoxon rank-sum, and chi-square tests were applied, where appropriate. Correlation of 2 continuous variables was examined with Spearman test. A logistic regression model was created for analyzing echocardiographic response. Kaplan-Meier curves were constructed to compare event rates among tertiles and formally assessed by using log-rank testing. Cox proportional hazards models were used for cumulative risk assessment of end points in the tertiles of 6MWT and physical activity, adjusted for covariates significantly associated with 1-month daily activity tertiles (age, coronary artery bypass graft surgery, hypertension, ischemic cardiomyopathy, and diuretics) and traditional risk factors (gender, coronary artery disease, diabetes, usage of cardiovascular medications, baseline LVEF, and creatinine level). During the multivariate modeling, the significant univariates (for HF hospitalization end point, gender, CAD, usage of diuretics, digoxin, and baseline creatinine; for composite end point, gender, diuretics, and creatinine) were included. All statistical tests were considered significant at p ≤0.05.
Results
Regarding the baseline characteristics of the entire study cohort, it was a typical CRT population ( Table 1 ). Significant differences in device-measured activity levels were seen for the summarized data between visits. Specifically, there was increase in median activity at 3 months (168 min/day [96 to 237], p = 0.001) and at 6 months (162 min/day [87 to 237], p = 0.011) compared with 1 month (135 min/day [72 to 210]) in the entire cohort. The 6MWT also showed significant improvement from baseline during subsequent follow-up visits (1 month: 320 m [380 to 350]; 3 months: 332 m [256 to 402], p = 0.007; 6 months: 338 m [274 to 421], p <0.001). Significant correlation between 6MWT and device-based physical activity data was observed at each visit. The correlation between summarized device diagnostic activity data and 6MWT was observed at 1 month (R = 0.407, p <0.01), at 3 months (R = 0.358, p <0.01), and at 6 months (R = 0.392, p <0.01) of visit. The patients were followed up for median 18 months (0-44 months, interquartile range 11 months).
| Characteristic | Activity (min/day) | p Value | ||
|---|---|---|---|---|
| <72 (n = 54) | ≤72 and <210 (n = 55) | ≥210 (n = 55) | ||
| Age (yrs) | 72.55 ± 9.69 | 69.6 ± 12.56 | 60.81 ± 12.94 | <0.001 |
| Women | 10 (18.9) | 12 (27.3) | 14 (24.6) | 0.578 |
| NYHA class III | 38 (77.6) | 41 (80.4) | 42 (75.0) | 0.923 |
| NYHA class IV | 2 (4.1) | 2 (3.9) | 4 (7.1) | 0.923 |
| Biventricular upgrade of pacemaker device | 30 (56.6) | 24 (43.6) | 24 (42.1) | 0.252 |
| LVEF (%) | 25.04 ± 6.92 | 25.3 ± 5.93 | 24.82 ± 6.89 | 0.939 |
| Coronary bypass | 27 (50.9) | 25 (45.5) | 10 (17.5) | < 0.001 |
| Chronic atrial fibrillation | 14 (26.4) | 13 (23.6) | 9 (15.8) | 0.372 |
| Diabetes mellitus | 25 (47.2) | 21 (38.2) | 18 (31.6) | 0.244 |
| Hypertension | 44 (83.0) | 41 (74.5) | 33 (57.9) | 0.012 |
| Ischemic cardiomyopathy | 37 (69.8) | 33 (60) | 22 (38.6) | 0.006 |
| Percutaneous coronary intervention | 15 (28.3) | 14 (25.5) | 11 (19.3) | 0.528 |
| Valve surgery | 10 (18.9) | 11 (20) | 6 (10.5) | 0.334 |
| ACE/ARB | 43 (81.1) | 44 (80) | 52 (91.2) | 0.199 |
| Aldosterone antagonist | 14 (26.4) | 18 (32.7) | 17 (29.8) | 0.773 |
| β Blockers | 48 (90.6) | 49 (89.1) | 52 (91.2) | 0.927 |
| Digoxin | 15 (28.3) | 17 (30.9) | 10 (17.5) | 0.227 |
| Diuretics | 48 (90.6) | 46 (83.6) | 40 (70.2) | 0.020 |
To assess the predictive value of the device-based physical activity and 6MWT data, we substratified the patients into tertiles. Patients with higher levels of activity had a significantly improved clinical outcome comparing with patients with tertile of lower level of activity.
The time to first HF hospitalization was examined during a follow-up period of 3 years. In the entire cohort, 20.5% (n = 45) were hospitalized over 3 years: 30.9% in the lowest tertile of device-based physical activity compared with 13.7% in the highest activity tertile (p = 0.026). Patients with higher levels of device-based activity had a better event-free survival with respect to time to first HF hospitalization compared with the lowest tertile (p = 0.004) by log-rank test (see Figure 2 ). Kaplan-Meier survival method demonstrated superior event-free survival with respect to time to first HF hospitalization in patients with a longer 6MWT (p = 0.001).




