Fig. 1.1
Schematic representation of the primary heart tube (brown) and the secondary added myocardium derived from the second heart field (yellow), including differential genes and proteins expressed in the second heart field. The second heart field can be divided into an anterior heart field and a secondary heart field at the anterior pole of the heart, and a posterior heart field at the venous pole of the heart. At the venous pole of the heart, the proepicardial organ (PEO) is also derived from the posterior heart field, and is the source of the epicardium and epicardium-derived cells. Neural crest cells (depicted in dark blue) migrate to the heart and enter the heart at both the arterial and venous pole. AVC atrioventricular canal, CV cardinal veins, CCS central conduction system, DOT distal outflow tract, LV left ventricle, OFT outflow tract, PAA pharyngeal arch arteries, POT proximal outflow tract, PV pulmonary veins, RV right ventricle, SAN sinoatrial node, SV sinus venosus
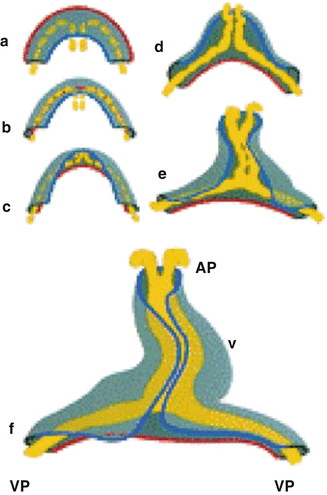
Fig. 1.2
Transformation of the flat cardiogenic crescent into a cardiac tube is displayed. During this process, the red outer contour of the myocardial crescent (gray) folds around the fusing endocardial vesicles (yellow) and passes the blue inner contour of the crescent, thereby forming the cardiac tube. AP anterior pole, VP venous pole, V future ventricle [Adapted from AFN Moorman et al., Development of the cardiac conduction system. Circulation Research 1998; 82:629–644. With permission from Wolters Kluwer Health]
Chamber differentiation occurs during further rightward looping of the heart tube, which results in positioning of the ventricles and the outflow tract of the heart in an anterior/ventral position, and of the atria in a dorsal/posterior position (Fig. 1.3). Transcriptional regulators (Nkx2-5, Tbx1, Tbx2, Tbx3, Tbx5, GATA4, Irx3 along with many others) and signaling pathways (including Notch, WNT, Bone Morphogenetic Protein [BMP], and Retinoic Acid) control chamber differentiation and formation of septal structures, the valves, and the great arteries [2, 3]. Electrical activity occurs early during development of the heart in conjunction with further differentiation of the simple heart tube into a four-chambered structure [4].
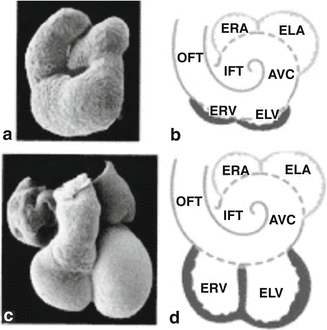

Fig. 1.3
Scanning electron photomicrographs (a and c) and schematic representations (b and d) of a 3-day embryonic chicken heart, where the first signs of the ventricles emerge (a and b), and of a 37-day embryonic human heart with clearly developed ventricles (c and d). ERA embryonic right atrium, ELA embryonic left atrium, ELV embryonic left ventricle, ERV embryonic right ventricle. The atrial segment is indicated in blue; the ventricular segment, in red; and the primary heart tube, encompassing the flanking segments, IFT, AVC, and OFT, as well as the atrial and ventricular parts, in purple [Adapted from AFN Moorman et al., Development of the cardiac conduction system. Circulation Research 1998; 82:629–644. With permission from Wolters Kluwer Health]
There has been much controversy with regard to the origin of the specialized myocardial tissue that leads to the development and expression of the conduction system. Current understanding suggests that cardiac myocytes, rather than neural crest cells, for example, are the progenitors of specialized conduction tissue. These findings were primarily supported by retroviral reporter gene transfection lineage studies [5–9]. The exact factors dictating this differentiation and development, however, remain to be elucidated, but it appears that neuregulin plays a crucial role in this differentiation process [10–16].
In a brief review in 1976, Wenink and colleagues proposed that there were four rings of specialized tissue in the embryo that could be distinguished from the surrounding myocardium once looping of the heart had occurred [17]. These four rings (Fig. 1.4) were thought to mark transitional zones of the heart and included: the sinoatrial ring, between the sinus venosus segment and the primitive atrium; the atrioventricular ring, between the primitive atrium and primitive left ventricle; the primary ring or fold, that separates the primitive left ventricle from the primitive right ventricle; and the ventriculoarterial ring, at the junction of the primitive right ventricle and the truncus or putative outflow tract of the heart [1] (Fig. 1.3). It is thought that during completion of looping of the primitive heart tube, these four rings come together in the inner curvature of the heart and with further differentiation; part of this tissue loses its specialized character. What remain of the rings become the definitive elements of the mature conduction system. According to this theory, the sinoatrial ring contributes to the formation of the sinoatrial node; both the sinoatrial ring and the atrioventricular ring contribute to the atrioventricular node. The primary ring gives rise to the His bundle and bundle branches while the ventriculoarterial ring regresses almost entirely.
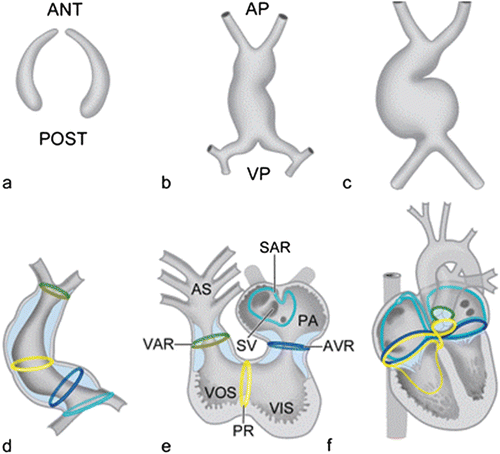

Fig. 1.4
Schematic representation of the bilateral formation of the cardiogenic plates, which are derived from the splanchnic mesoderm (a). The bilateral plates fuse and form an initially straight heart tube (b) that starts looping to the right (c, d). After looping, the so-called transitional zones or rings can be recognized in the heart that are positioned in between the putative cardiac chambers, i.e., the sinoatrial transition (SAR), the atrioventricular transition (AVR), the primary ring (PR), and the ventriculoarterial transition (VAR) (e). Position of these rings during further cardiac development (f). Ant anterior, AP arterial pole, AS aortic sac, PA primitive atrium, post posterior, SV sinus venosus, VIS ventricular inlet segment, VOS ventricular outlet segment, VP venous pole. a–c [Adapted from Gittenberger-de Groot AC, Bartelings MM, Deruiter MC, Poelmann RE. Basics of cardiac development for the understanding of congenital heart malformations. Pediatric Research 2005;57:169–176. With permission from Nature Publishing Group]
Studies in the 1990s used the expression pattern of a neurofilament-like protein as a marker for the developing conduction system. The presence of neurofilament-like protein was used to demonstrate a ring at the sinoatrial and atrioventricular junctions, and in ventricular components of the developing conduction system, which were distributed in the ventricular subendocardium and connected to the atrioventricular ring [18–22]. In contrast to the theory that local cells undergo specialized differentiation, other studies suggest that conduction tissue cells (of the rabbit heart, for example) may originate from a population of neural crest-derived cells migrating from the branchial arches into the developing heart [20].
As these conflicting theories continued to be investigated, several immunohistochemical and molecular markers for cardiac conduction system development were used to support the hypothesis that conduction system cells differentiate from local cells. Even though none of the immunohistochemical markers are truly specific for labeling specialized conduction system cells, supportive evidence seemed to favor the “four ring theory.” Using a monoclonal antibody to HNK1 antigen, for example, investigators demonstrated findings consistent with the notion that rings of conduction system tissue exist and undergo further differentiation (Fig. 1.5). HNK1 is predominantly expressed in the developing sinoatrial and atrioventricular junction of the conduction system, and the expression pattern seems to correspond with the rings described early on by Wenink. In human embryos, antibodies to HNK1 antigen stains the sinoatrial node, the internodal myocardium in the right atrium, the right atrioventricular ring with a future posterior and anterior atrioventricular node, a retroaortic ring, the His bundle, and the bundle branches. It appears that the myocardium surrounding the primitive pulmonary veins also demonstrates transient staining of HNK1 [23].
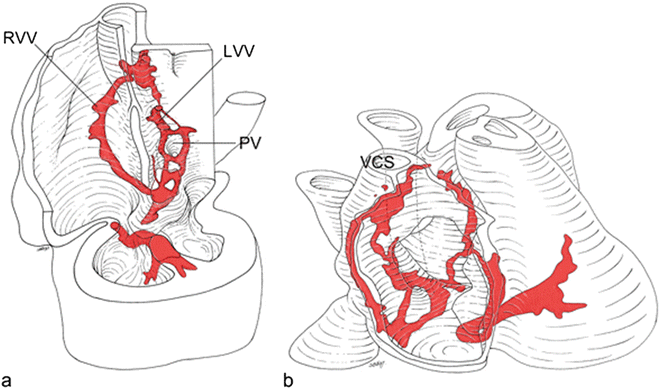

Fig. 1.5
HNK1 stains the sinoatrial node, the internodal myocardium in the right atrium, the right atrioventricular ring with the posterior and anterior atrioventricular nodes, a retroaortic ring, the His bundle, and the bundle branches in human embryos. Furthermore, the myocardium surrounding the primitive pulmonary vein demonstrates transient staining. RVV right venous valve, LVV left venous valve, PV pulmonary veins, VCS superior vena cava [Adapted from Blom NA, Gittenberger-de Groot AC, DeRuiter MC, Poelmann RE, Mentink MMT, Ottenkamp J. Development of the cardiac conduction tissue in human embryos using HNK-1 antigen expression—Possible relevance for understanding of abnormal atrial automaticity. Circulation 1999;99:800–806. With permission from Wolter Kluwers Health]
Podoplanin is a 43-kd, mucin-type transmembrane glycoprotein that is found outside the heart in several organs and tissues [24–32], such as osteoblasts, the nervous system, epithelia of lung, eye, esophagus, and intestine, mesothelium of the visceral peritoneum; the podocytes of the kidney; and lymphatic endothelium [33–36]. It is thought that podoplanin expression in the developing heart is a marker for the developing sinus venosus myocardium, supporting its development from the posterior heart field. Podoplanin is expressed in the areas that are in close contact with the sinoatrial nodal myocardium and in the underlying mesenchyme adjacent to the cardinal veins. It appears that podoplanin-positive mesenchyme differentiates into myocardium that stains negative for Nkx2.5. During cardiac development, podoplanin is expressed in myocardium along bilateral cardinal veins and in both the right-and left-sided sinoatrial nodes. This expression is maintained on the right as part of the right sinus node and right-sided venous valves, at the base of the atrial septum, the posterior atrioventricular canal, the atrioventricular nodal region, and the His-Purkinje system; it is opposed by the expression of Nkx2.5. Also, during later developmental stages, podoplanin is expressed in the pulmonary veins. In podoplanin negative mice, myocardial components around pulmonary veins are reduced and there is underdevelopment of the atrial septum [28, 37]. It appears that podoplanin plays a critical role in myocardial tissue associated with the sinus node and that abnormal epithelial-to-mesenchymal transformation of the coelomic epithelium due to up-regulated E-cadherin and down-regulated RhoA impose abnormalities in the formation of cells that form the sinus venosus [28].
Even though the development of anatomical structures supporting the specialized conduction system offers insight into genesis of the conduction system, functionally, the development of impulse generation and propagation remains to be fully understood. In the mature heart, the sinoatrial node is the primary pacemaker of the heart, and impulse propagation occurs through the atrioventricular node and specialized His-Purkinje system. Impulse propagation, itself, can be further divided into fast, as in the His-Purkinje system, and slow as seen in myocardial tissue, and slower yet, as seen in the atrioventricular node [6]. Different animals reveal complex variations in the organizational and functional components of the conduction system [7, 8, 38–40]. For example, the study of the chick embryo allowed understanding of a pacing generator around 25–35 h of development in the posterior most part of the tubular heart [41]. Similarly, pacing activity is noted around 7.5 days and 21 days in mice and humans, respectively [42]. At this time, the heart consists of a simple heart tube and the initial contractions are slow and rhythmic [43], but establish unidirectional flow and posterior to anterior polarity [44–48]. These peristaltic contractions can be recorded, inscribing a sinusoidal ECG [49].
Furthermore, there appear to be transient expression of key transgenic markers, timed chronologically, that determine developmental fate of myocardial cells. For example, the heart tubes in zebrafish, chicks, and mice appear to have retinoic acid-sensitive markers along the heart tube that dictate formation of atrial tissue [50]. Retinoic acid appears to control atrial-specific gene expression and exclusion of retinoic acid from ventricular tissue precursors seems essential for correct specification of the ventricular muscle development. In addition, transmembrane hyperpolarization-activated cyclic nucleotide-gated family of ion channel subunits plays a key role in impulse generation supporting pacemaker activity, both in the embryo and the adult human heart [49, 51–53]. Other genes also play a role in impulse generation as can be seen in studies that show that knock-out of the NaCa exchanger gene causes mortality due to inhibition of pacing function in the tubular heart [54]. Along with further differentiation, the developing atrial and ventricular myocardial cells acquire high conductance gap junctions that can then support rapid transmission of an electrical impulse by rapid proliferation and up-regulation of genes. These working myocardial cells have increased mitochondria and increased sarcomere components.
In contrast to rapidly proliferating myocardial tissue, cells in the atrioventricular canal area retain their slow proliferation rates, and also retain their “embryonic-like” mode of conduction, which is much slower [51, 55, 56]. In association with chamber formation, slow wave propagation producing peristaltic contractions are replaced by rapid depolarizations (and contractions) of cells of the atrium and ventricles, inscribing an ECG that resembles the one of the mature heart. These changes seem to occur in parallel with anatomical looping of the heat tube. Differential expression in conduction velocities of the conduction system components in the mature heart accompanies looping. These structural changes parallel a delay in conduction time in the mature atrioventricular node [57–59]. In fact, in the adult myocardium, the impulse proceeds from the sinus node to the crux of the heart at 0.1–1.0 m/s and is slowed at the atrioventricular node to 0.01–0.05 m/s, increasing its velocity to 2–4 m/s in the His-Purkinje system, with a decrease to 0.3–1.0 m/s in the ventricular myocardium [4]. Thus, sequential contraction of the atrial and ventricular chambers in higher species is dependent on the specific functional development of atrioventricular delay [5, 6, 16, 43, 60, 61]. This delay can be seen at 42 h of development in the chick and at 8 and 25 days in the mouse and human [6, 7], respectively. Furthermore, in the looping heart, there are two other areas of relatively slow conduction: the sinoatrial area and the outflow tract area. This slow conduction is associated with the expression of connexin 45, which is characterized by high voltage sensitivity and low permeability [62–65]. Knock-out mice of the connexin 45 gene result in death from heart block at looped, tubular stages of heart development [66, 67].
The His-Purkinje system is the last component of the conduction system to differentiate. In mammals, differentiation of the His-Purkinje system is quite advanced, resulting in markedly efficient and coordinated myocardial activation and associated myocardial contraction [5, 8, 68]. Retroviral lineage studies suggest that central and distally located components of the His-Purkinje system differentiate separately, but then link together during development [7]. In 1999 and 2000, differentiation of the His-Purkinje system in ventricular myocytes was found, in the chick embryo, to be induced by endothelin-1, secreted from adjacent coronary arterioles [69, 70]. This particular finding was not noted in the mouse, however. Similarly, some in vitro evidence suggested that neuregulin-1 played a role [15, 71, 72] in His-Purkinje development. In addition, cellular studies observed that there is a switch in activation sequence in the developing heart. The emergence of the mature His-Purkinje system in the developing chicken embryo had been studied using anti-polysialylated neural cell adhesion molecule (PSA-NCAM) and the HNK1 antibody against a sulfated carbohydrate epitope (antigen). The appearance of the mature form of the His-Purkinje system coincided with the onset of the mature electrophysiological patterns of ventricular activation. These data suggested that, at the completion of ventricular septation, the His-Purkinje system undergoes critical structural and functional transitions that impacted the global pattern of impulse conduction and contraction of the developing four-chambered heart [73, 74]. Using cardiac conduction system-lacZ line of reporter mice, several investigators tested the ability of endocardial-derived and secreted (paracrine) factors to convert contractile cardiomyocytes into conduction system cells. It appeared that neuregulin-1, a growth and differentiation factor essential for ventricular trabeculation was sufficient to induce ectopic expression of a lacZ conduction marker. In the mouse model, this inductive effect of neuregulin-1 was restricted to a window of sensitivity between 8.5 and 10.5 days fertilization. Thus, it appeared that endocardial-derived neuregulins may be responsible for inducing murine embryonic cardiomyocytes to differentiate into cells of the conduction system [15]. In a similar manner, Gassanov et al. described differentiation of atrial-derived cardiomyocytes to a pacemaker-like phenotype induced by endothelin-1, but not associated with neuregulin [72].
Cellular Development of “Nodal” Phenotype
In the mature heart, the atrioventricular nodal myocytes display a variety of embryonic characteristics. Despite these characteristics, nodal cells are poorly distinguishable from surrounding myocardium in the embryonic heart. During gestation and development, nodal cells retain organized actin and myosin filaments and a poorly developed sarcoplasmic reticulum. Nodal cells also continue to express different structural and cellular markers, which are species specific. Several classes of markers have been identified including connexins, specific contractile proteins, desmin, and neurofilaments. These specific markers provide an opportunity for the study of conduction system development. During development, unique characteristics of nodal cells include the expression of higher amounts of calcium-release channel/type-1 inositol triphosphate receptor, gamma enolase, alpha 1 and alpha 2 units of the sodium pump, G-protein alpha subunit, and angiotensin II receptor [26, 75–86]. The role of these differences is unclear at this time. Antibodies to carbohydrate markers such as the polysialylated neural cell adhesion molecule and HNK1 have been used to study the development of specific regions of the specialized conduction tissue. The role of some of these key factors is reviewed below.
The T-Box Family of Transcription Factors
The T-box transcription factors Tbx2 and Tbx3 are expressed in the cardiac inflow tract, the atrioventricular canal, the outflow tract, and inner curvature of the heart during development. These factors are transcriptional repressors of chamber formation. Both Tbx2 and Tbx3 suppress the genes Nppa and connexin 40, present in working myocardium [55, 76, 87–89]. In general, expression of Tbx2 and Tbx3 is observed in slow-conducting areas, but also in the His bundle and the proximal part of the bundle branches. The expression of Tbx2 decreases from early fetal stages, whereas the expression of Tbx3 increases.
In the developing heart, expression of Tbx3 is observed in the sinoatrial node and atrioventricular node, but also in the internodal myocardium, in the atrioventricular cushions and in the His bundle and proximal bundle branches [88, 89]. Homozygous Tbx3-mutant mice display a syndrome known in humans as ulnarmammary syndrome and display early embryonic mortality, presumably due to severe compromise of the yolk sac [90]. The role of Tbx3 in controlling the sinoatrial node gene program has also been described [88, 89]. Tbx3 is expressed in the developing and mature sinoatrial node and is required to suppress the expression of genes regulating atrial differentiation. Furthermore, Tbx3 can induce ectopic pacemaker sites in the atria [88, 89].
The T-box transcription factor Tbx5 is expressed in the developing the atrioventricular node, His bundle, and bundle branches [85]. Mice lacking Tbx5 display a cardiac phenotype that resembles the Holt–Oram syndrome, including atrial septal defects and conduction system abnormalities [2]. Tbx5 targets atrial naturetic factor (ANF) and connexin 40 as part of the fast-conducting components of the conduction system. In mice, Tbx5 haplo-insufficiency causes a maturation failure of conduction system morphology and function [85]. Tbx5 is required for connexin 40-independent patterning of the cardiac conduction system and it is thought that the electrophysiologic defects in Holt–Oram syndrome reflect a developmental abnormality of the conduction system [82]. Tbx18 is expressed in the sinus horns and is likely essential for the formation of the sinus venosus. In mice that are deficient for Tbx18, formation of the sinus venosus is disturbed [87].
Homeodomain Transcription Factors
The homeodomain transcription factor Nkx2.5 is expressed early in development, in the cardiogenic mesoderm and is present throughout the developing heart [91]. As part of an ongoing chamber formation program, Tbx5 and Nkx2.5 stimulate a variety of cardiac genes. During development certain regions in the linear heart tube remain embryonic in nature and do not develop into working chamber myocardium due to the presence of Tbx2. Thus, it appears that Nkx2.5 and Tbx2 form a repressor complex that suppresses genes that promote a chamber differentiation program. Tbx2 is expressed in the primary myocardium of the inflow tract, atrioventricular canal, and outflow tract. It appears that Tbx2 competes with Tbx5, and when Tbx2 is expressed in conjunction with Nkx2.5, it seems to act as a repressor of further differentiation.
The expression of Nkx2.5 is elevated in the differentiating atrioventricular conduction system, compared to its expression in the adjacent working myocardium. This expression correlates with the recruitment of cells to the developing atrioventricular conduction system [92]. In Nkx2.5 haplo-insufficient mice, there is hypoplasia of the atrioventricular node and His bundle, and the number of peripheral Purkinje fibers is significantly reduced [93–95].
Cardiac phenotypes of mutations in Nkx2.5 in mouse models resemble those in humans and include conduction defects [96]. It is known that Nkx2.5 is not expressed in posterior heart field-derived myocardium, including the sinoatrial node and the sinus venosus [87]. Furthermore, Nkx2.5 interacts with the connexin 40 promoter region and mice lacking Nkx2.5 demonstrate a significant decrease in connexin 40 expressions [97]. Nkx2.5 can form a complex with the transcription factor Tbx2 that is able to suppress ANF promoter activity in the atrioventricular canal, which may be a mechanism that helps to regulate some of the sites of chamber formation in the developing heart [91]. Nkx2.5 can also bind to Tbx5. This complex is an essential component for the activation of the atrial naturetic factor gene.
The homeodomain transcription factor Msx2, a downstream target of Pax-3/splotch (which is a key player within early cardiac neural crest development), is expressed in the developing central conduction system, but not the peripheral Purkinje fibers, in the chick. However, no abnormalities in the cardiac conduction system have been observed in Msx2-mutant mice [95, 98].
The homeobox gene Hop is strongly expressed in the atrioventricular node, His bundle, and bundle branches of the adult cardiac conduction system and Hop-null mice demonstrate conduction defects below the atrioventricular node, related to decreased expression of connexin 40 [99].
The homeodomain transcription factor Shox2 is expressed in the embryo in the craniofacial region, limbs, brain, and heart [100, 101]. In the heart, Shox2 can be detected early in the posterior region of the primitive heart tube. During further development, Shox2 is expressed in the sinus venosus myocardium, which includes the sinoatrial nodal region and the venous valves; expression is also observed in the primitive left and right bundle branches. Shox2 knock-out mice die between 11.5 and 13.5 days postfertilization and show severe hypoplasia of the sinus venosus myocardium of the posterior heart field, including a decreased size of the sinoatrial node region and hypoplastic venous valves. When Shox2 is absent in knock-out mice, aberrant expression of connexin 40, connexin 43, and Nkx2.5 is observed within the sinoatrial node, indicating abnormal differentiation of the sinoatrial node as well as disturbed pacemaker function. This finding is also noted in the node in zebrafish embryos [100]. Given these findings, it appears that Shox2 is important in recruiting sinus venosus myocardium, including the sinoatrial nodal region.
The bicoid-related homeodomain transcription factor Pitx2c is involved in directing left/right identity in the heart at the venous pole [83] and is probably involved in suppression of left-sided sinus node formation, as Pitx2c-deficient fetuses form sinoatrial nodes in both the right and left atrium [102, 103].
Id Family of Transcriptional Repressors (Helix-Loop-Helix Containing Transcriptional Repressors)
Early in cardiac development, the temporal and spatial expression of Tbx5 supports specification of cells for the conduction system. Tbx5-dependent expression of connexin 40 and presumably other molecules are required for the critical electrophysiologic properties of these cells. It is thought that Tbx5 directs the expression of certain genes, such as those for connexin 40, in the mature conduction system, after the primitive atrioventricular node, left bundle branch and right bundle branch have assumed their adult structures. This observation may explain why some Holt–Oram patients, for example, or Tbx5del/+ mice show an evolution of conduction system disease with age [90, 91].
The gene Id2 has been identified by serial gene expression analysis (SAGE) as having ventricular conduction system expression and is a downstream target of Tbx5 and Nkx2.5. Id2 negative mice demonstrate ECG features of abnormal interventricular conduction, such as left bundle branch block in newborn and adult knock-out mice. Furthermore, intracardiac recordings are consistent with abnormal intraventricular conduction within the bundle branches [85]. In situ hybridization demonstrated that Id2, expressed in the conduction system in wild-type hearts, is not expressed in compound Tbx5/Nkx2.5 hearts, indicating that ventricular conduction system-specific expression of Id2 is dependent on Nkx2.5 and Tbx5. These findings support a link between a patterning abnormality of the developing conduction system and a functional abnormality of the mature conduction system [85].
Basic Helix-Loop-Helix (bHLH) Transcription Factors
Fate mapping analysis has revealed that Mesp1 is expressed in almost all of the precursors of the cardiovascular system, including the endothelium, endocardium, myocardium, and epicardium. Mesp1-nonexpressing cells were found to be restricted to the outflow tract cushion and along the interventricular septum. When the interventricular cells were examined by using the pattern of beta-galactosidase activity, approximately 20 % of the ventricular conduction cells within the intraventricular septum correspond to Mesp1-nonexpressing cells. These data suggested that the ventricular conduction system is of heterocellular origin [104].
The GATA Family of Transcription Factors/Zinc Finger Subfamilies
The GATA family is a relatively small family of transcription factors. For three of the six known vertebrate GATA transcription factors, a role in cardiogenesis has been identified: these include GATA4, GATA5, and GATA6 [105]. Expression of GATA4 is present in both the adult and embryonic heart, and its disruption results in cardiac dysmorphogenesis with early embryonic mortality [87]. A significant interaction among the different transcription factors was shown in a study that demonstrated that, next to Tbx3 and Nkx2.5, the connexin 40 promoter is also modulated by the cardiac transcription factor GATA4 [97]. In addition, GATA4 is expressed in Purkinje fibers of the adult chick heart [106]. GATA5 mRNA is observed in the pre-cardiac mesoderm of the primitive streak embryo. In the embryonic heart, there is expression of the GATA5 gene in the atrial and ventricular chambers that, during further development, becomes restricted to the atrial endocardium [107]. Furthermore, GATA5 is expressed in the endocardial cushions and in the cardiac conduction system, in the sinoatrial node, atrioventricular node, bundle of His, and left and right bundle branches [84]. Interestingly, the GATA5 gene is expressed in a dynamic fashion over time in the septum transversum and in the epicardial organ of the mouse and avian heart, giving rise to the (GATA5-expressing) epicardium [84]. The cGATA6 gene enhancer specifically marks components of the developing cardiac conduction system and atrioventricular node [78, 108], but not the more distal components of the cardiac conduction system. Expression of cGATA6 remains visible in the mature cardiac conduction system.
MinK/lacZ Knock-In/Knock-Out
The minK gene (also known as IsK and KCNE1) encodes a 129-amino-acid protein that modifies transmembrane electrical currents in the heart resulting from expression of the genes HERG and KvLQT1 [109]. Mutations in both HERG and especially KvLQT1 that encode the structural subunits for the channels involved in the cardiac delayed rectifier currents IKr and IKs, respectively, are the most common causes of congenital long-QT syndrome (LQTS) [109]. Disruption of the minK gene and integration of the lacZ gene results in β-galactosidase expression under the control of endogenous minK regulatory elements, which has been used to study the expression pattern of minK in mice. Disruption of the minK gene causes inner ear defects and QT interval prolongation in bradycardic conditions, the combination of which is known in the human as the Jervell and Lange-Nielsen syndrome [110]. MinK–/– myocytes lack the delayed rectifier current IKs and demonstrate significantly reduced IKr, which indicates a role of minK in modulating both rectifier currents [109]. The spatial expression of minK-lacZ in the adult mouse heart has been shown to be coincident and closely related to the conduction tissue. The expression of minK-lacZ has been used to trace the embryonic development of the conduction system. Expression of minK-lacZ was first seen on the eighth embryonic day in the mouse. Subsequently, discrete rings were found at the sinoatrial, atrioventricular, interventricular, and ventricular–arterial junctions, and with time, the expression became restricted to boundary regions of the heart, such as the hinges of the leaflets of the pulmonary and aortic valves, the atrioventricular rings, and the venous valves, but was also noted in the definitive conduction tissue. In the postnatal mouse heart, areas retaining minK-lacZ positivity outside of the definitive conduction tissues were thought to designate sites of origin of abnormal cardiac rhythms, suggesting that ectopic foci may derive from tissues that share a common developmental pathway with the definitive conduction system [111]. These observations suggest that the boundary regions between compartments, along with the atrioventricular conduction axis, share common developmental pathways and may support a certain arrhythmia expression later in life. Expression of minK-lacZ was not present at the site of the pulmonary veins [111].
Cardiac Conduction System lacZ Insertional Mutation
In 2000, it was noted that the random insertion of a lacZ gene into the murine genome unexpectedly resulted in a mouse line (named Cardiac Conduction System-LacZ [CCS-LacZ]) with expression of lacZ in the (developing) conduction system of the heart. Genetic mapping demonstrated that the transgene inserted into a regulatory region on mouse chromosome 7, altering transcription of several nearby genes including Slco3A1 [10–15, 47].
Regulatory elements from the gene Slco3A1 influenced cardiac conduction system-restricted reporter gene expression [112]. Members of the Slco family encode for organic anion-transporting polypeptides that mediate transport of natural substances (such as prostaglandins, bile salts, thyroid, and steroid hormones) as well as exogenous drugs (including digoxin, angiotensin-converting enzyme inhibitors, HMG-coenzyme A reductase inhibitors, methotrexate, and rifampin) across the cell membrane [113]. Considering the extent of the recombination observed in the CCS-LacZ model, it was thought that it would be likely that regulatory elements from more than one gene are involved [112]. In the CCS-LacZ mouse LacZ is expressed in all components of the developing cardiac conduction system, including the right and left venous valves and septum spurium of the sinus venosus and the putative sinoatrial node, the left and right atrioventricular ring, His bundle, bundle branches, and Purkinje fibers. CCS-LacZ is also expressed in the moderator band of the right ventricle, Bachmann’s bundle, the retroaortic root bundle, and in the myocardial sleeve that develops around the pulmonary vein, areas related to arrhythmias in adults. Findings in several models support the hypothesis that the occurrence of cardiac arrhythmias in the heart, especially on the left atrial side, may be related to persistent or reactivated areas of developing cardiac conduction system [114–116].
CCS-LacZ expression was also noted in intraluminal endothelial cells, which are thought to be linked to the secretion of endothelial-derived factors involved in induction of cardiomyocytes to acquire a conduction system phenotype. Indeed, the endothelial paracrine factor neuregulin-1 has been demonstrated to induce ectopic expression of CCS-LacZ and, therefore, may play a critical role in recruitment of cells to the cardiac conduction system [15]. Timing of exposure to the endothelial factors may be crucial, as the inductive effect of neuregulin in the CCS-LacZ mouse was restricted to a window of sensitivity between E8.5 and E10.5 [15]. In the adult mouse heart, using serial sections of CCS-LacZ hearts, connexin 40 immunostaining (marking ventricular cardiac conduction system cells) could be co-localized with CCS-LacZ transgene expression in the atrioventricular node, His bundle, bundle branches, and subendocardial Purkinje fibers along the interventricular septum. In contrast to the developing heart and neonatal heart, cardiac CCS-LacZ expression was no longer present within the sinoatrial node in the adult mouse heart [117].
The Hyperpolarization-Activated Cyclic Nucleotide-Gated Cation (HCN) Channel Family
Four genes that encode HCN channels have been identified: HCN1, HCN2, HCN3, and HCN4. HCN channels carry an inward current, which is the depolarizing Na/K current if, that underlies cardiac pacemaker activity. In the adult heart, both HCN2 and HCN4 are expressed. During development, HCN4 is expressed as early as E7.5 in the cardiac crescent [118, 119]. Interestingly, in the early heart tube, using optical mapping studies in the chick [120, 121], expression is observed bilaterally in the sinus venosus. Later in development, expression of HCN becomes asymmetrical and restricted to the right atrium, at the site of the developing sinoatrial node [118]. In the postnatal and adult heart, HCN4 is highly expressed in the sinoatrial node [118, 119]. HCN4 knock-out mice die between E9.5 and E11.5. These knock-out mice do not display mature pacemaker potentials, and thus, it is thought that HCN4 channels are required for proper pacemaker function of the sinoatrial node [119]. The expression pattern of HCN4 overlaps with the expression of markers of the posterior heart field, such as podoplanin and Shox2. The expression of HCN4 reflects the sinus venosus myocardium of the posterior heart field and becomes restricted to the sinoatrial node [118]. HCN2 is expressed in a broader distribution pattern than HCN4 and includes the ventricular myocardium, but is also moderately expressed in the sinoatrial node [118].
Connexins
The transmission of the electrical action potential is thought to occur primarily through gap junctions. Gap junctions are aggregates of membrane channels, composed of protein subunits named connexins that are encoded by a multi-gene family. Connexins hexamers make up connexons that then form the gap junction. Four different connexins are expressed in the mammalian heart including connexin 30.2, 40, 43, and 45. In the early myocardium, both number and size of gap junctions are small but they increase during development. The number of gap junctions remains scarce in the developing sinoatrial node and the atrioventricular node. The low abundance of connexin expression in the two nodes corresponds to their slow conduction velocities. This difference in connexin concentration has been an important marker for nodal-specific tissue. An abrupt rather than gradual increase in the number of gap junctions is found at the transition zone of nodal tissue to working myocardium. This boundary is thought to be due to a decrease in the number of nodal cells towards the atrial working myocardium rather than a gradient due to a change in molecular phenotype. Fast-conducting cardiac tissues in the atria express connexin 40 [122, 123] and slower conducting working myocardium express connexin 43 [124]. Connexin 45 seems to play a crucial role in delineating the conduction system during development and is seen in slow-conducting pathways, including the sinoatrial node and atrioventricular node during development [62–65, 124]. The expression of the different connexins varies among species but does provide an insight into the interplay and non-static nature of gap junction expression during development. For example, connexin 40 can be detected early in the mouse heart, where it is present first in the primitive atria and primitive left ventricle, and also in the primitive right ventricle, but not in the AV canal and interventricular septum. During development, together with the development of the specialized conduction system tissue, expression of connexin 40 becomes restricted to atrial myocytes and the ventricular conduction system [123]. Connexin 40 knock-out mice display an increased incidence of inducible atrial arrhythmias, and significant conduction delay in the infra-His and distal atrioventricular nodal conduction [62, 63, 122, 123, 125–129].
Connexin 43, in contrast, is first detected in the primitive ventricle and, some, in the atria and its expression increases and is present in the adult ventricular (working) myocytes [122, 123, 128]. Connexin 43 knock-out mice die at birth because of developmental defects in the pulmonary outflow tract, presumably resulting from defective migration of cardiac neural crest cells to this region [129]. In addition, cardiac-specific deletion of connexin 43 results in sudden cardiac death from spontaneous ventricular arrhythmias at 2 months postnatally, which suggests an important role for connexin 43 with regard to maintenance of electrical stability in the heart [130].
Connexin 45 is expressed in all compartments of the linear heart tube, including the inflow tract, atrioventricular canal, and outflow tract. Expression of connexin 45 decreases throughout development and in the adult mouse heart, but remains present in the atrioventricular node, His bundle, and surrounding Purkinje fibers [62–65]. Connexin 45 knock-out mice demonstrate conduction block and die of heart failure [130].
Finally, connexin 30.2 slows impulse propagation through the atrioventricular node, which is important in preventing rapid conduction of an impulse into the ventricles [131–133]. In mice, in which the coding region of connexin 30.2 has been replaced by a lacZ reporter gene, a shortening of the QT interval by 25 % is seen [131].
Cytoskeletal Proteins
Nodal-specific developmental expression of contractile proteins such as myosin heavy chain and its isoforms, desmin and neurofilament, has been used to delineate the sinoatrial and atrioventricular nodes. However, inter-species variability in the staining of these markers does not produce sufficiently consistent data to draw definitive conclusions with relation to development or morphologic changes that are specific to the conduction system or its development and differentiation.
Atrioventricular Junction: Accessory Pathways/Mahaim Fibers
The atrial and ventricular myocardium including the atrioventricular canal is a continuous structure during embryogenesis of the heart tube and development of the four-chambered heart [134]. As demonstrated in the chick, accessory atrioventricular myocardial continuities may persist in the embryo until later stages, causing premature activation of the ventricles even after septation has occurred [135]. In normal adult cardiac conduction, the atrioventricular node-His bundle is the only functional atrioventricular conduction tract between the atria and ventricles. Rarely, accessory myocardial bundles or pathways connecting atrial and ventricular myocardial tissue persist, thus bypassing the insulating function of the atrioventricular groove [134] resulting in the well known in Wolff–Parkinson–White syndrome in humans [134]. A rare form of an accessory pathway is a right-sided accessory bundle with atrioventricular node-like conduction properties, at one time thought to be Mahaim fibers, but more correctly are atriofasicular fibers [116, 134, 135]. Data derived from the CCS-LacZ mouse demonstrate that the occurrence of these rare fibers may be related to the embryonic development of the right ventricular inflow tract. The development of the right atrial/right ventricular connection and concomitant outgrowth of the right ventricle results in a division of the primitive left and right ventricles. This division results in the development of the right ventricular moderator band that forms a right-sided atrioventricular continuity, similar to a Mahaim fiber. Electrophysiological experiments supported the presence of a slowly conducting right-sided atrioventricular pathway [116]. Other rare anomalous fibers that bypass the normal atrioventricular node-His-Purkinje axis are nodoventricular fibers atrioventricular node-ventricular connection) and fasciculo-ventricular (His bundle or right bundle connection) fibers that can cause pre-excitation and, rarely, reentry tachycardia. They are known as Mahaim fibers. These forms of pre-excitation can result in atrioventricular reentrant tachycardia (Chap. 4).
To date, there are two mouse models of Wolff–Parkinson–White syndrome. Mutations in the gene PRKAG2 (that encodes the γ-2 subunit of the AMP-activated protein kinase) seem to be associated with the expression of Wolff–Parkinson–White syndrome [136, 137] in humans. Mice that carry a mutation in the PRKAG2 gene display ventricular pre-excitation and a phenotype identical to humans with the familial form of ventricular pre-excitation [138]. Another form of pre-excitation has been demonstrated where the postnatal development of myocardial connections through the annulus fibrosus of the atrioventricular valves in mice overexpressing the PRKAG2 mutation occurs [139]. In this type of pre-excitation, there seems to be accumulation of excessive amounts of cardiac glycogen, and disruption of the annulus fibrosus by glycogen-filled cardiomyocytes [140, 141]. This form of pre-excitation is associated with myocardial hypertrophy.
A specific deletion of the gene ALK3 in the atrioventricular canal, coding for the type 1a receptor for bone morphogenetic proteins in the atrioventricular canal during development, causes ventricular pre-excitation, also, supporting the notion that this gene is important for normal atrioventricular junction development [80].
Epicardial inhibition studies demonstrate that reduced periostin expression at the atrioventricular junction, results in disturbed development of fibrous tissue at the atrioventricular junction, persistent atrioventricular myocardial connections with resulting ventricular pre-excitation, which may be another mechanism explaining Wolff–Parkinson–White syndrome [135].
In contrast to arrhythmias associated with accessory pathways, several other arrhythmias have been described that originate from the tricuspid and mitral valve junctions [142, 143] or around the atrioventricular annuli. Atrioventricular cells surrounding both the tricuspid and mitral annuli have been shown to resemble nodal cells in their cellular electrophysiology [144], and thus, could support arrhythmias similar to those intrinsic to the atrioventricular node.
In summary, evidence suggests that the specialized conduction system develops from further differentiation of local myocytes. The molecular signals for this differentiation are multiple, variable, interactive, and dose- and timing dependent. The exact stimulants for differentiation, selective cellular potency, and variable cell protein and channel expression and their roles in differentiation deserve further study.
Part II—Anatomy of the Mature Cardiac Conduction System
The specialized conduction system of the mature human heart consists of a single sinoatrial node, atrial and intranodal pathways, the AV node, and the His-Purkinje system, the latter includes the right and left bundle branches of the His-Purkinje system, and the peripheral His-Purkinje system. This section focuses on the anatomy of the conduction system and its relationship with the luminal working myocardium as both are developed, in parallel and in conjunction with each other.
All cells in the heart are capable of conducting an electrical impulse, but a special subpopulation of myocytes differentiates to support both generation and propagation of the cardiac impulse. Even though microscopic inspection provides considerable insight into the structure of the conduction system [145], this method is incomplete and does not fully define and delineate specialized tissue behavior with regard to intramyocardial behavior and interaction [146, 147].
In the mature heart, the sinoatrial (SA) node is the dominant pacemaker of the heart and lies in the right atrium at the superior vena cava/right atrial junction, one millimeter below the epicardium of the sulcus terminalis [148–150]. It was first described in the early 1900s [151]. The sinoatrial node has the shape of an inverted comma, descriptively containing a head, body, and tail [145, 152–155]; rarely, the sinoatrial node has a horseshoe-shaped structure [156]. It tapers both medially and laterally and bends backward towards the left and then downward [157]. Several authors document a paranodal area, where cells are of the node intermingle with atrial cells [158–160]. The sinoatrial node is supplied by a relatively large artery, which courses through and gives off branches to the sinus node and adjacent atrial myocardium. It originates from the right coronary artery about 55 % of the time and from the left circumflex artery in about 45 % of cases [156].
With regard to the atrial body itself, it is controversial whether preferential intranodal pathways exist [161, 162] because conclusive anatomic data is missing.
Even though evidence for preferential intra-atrial pathways is missing, there appears to be preferential conduction or impulse propagation that may be associated with the underlying anatomic differences in muscle density, muscle fiber orientation, and/or the thickness of the right atrial wall and its pectinate muscles. Some authors argue that “specialized pathways” consisting of aggregations and or concentrations of myocardial muscle fibers, bridge the SA and atrioventricular nodes or the right atrium to the left atrium. These authors propose three internodal tracts: the anterior, middle, and posterior internodal fibers. The anterior intermodal fibers are thought to have two components: Bachman’s Bundle, which bridges right and left atrium and “descending branches,” which descend in the intra-atrial septum. The middle internodal tracts also known as Wenchebach’s bundle are thought to arise from the posterior portion of the sinus node and then descend within the intra-atrial septum, anterior to the fossa ovale. And the posterior internodal tracts, also known as Thorel’s pathway, are thought to exit the sinus node posteriorly and then descend within the crista terminalis, traversing through the Eustachian ridge, entering the AV node, posteriorly, in the mouth of the coronary sinus [161, 162]. An alternate hypothesis is that intra-arterial conduction depends on gap junction density at the cellular level.
Because it was difficult to differentiate electrical myocardium from working myocardium, anatomically, in 1910, the German Pathological Society defined myocytes that were responsible for conduction to be associated with: 1—histologically discrete from adjacent contracting myocardium, 2—traceable from pathologic section to section, and 3—insulated to some degree from adjacent tissue by a fibrous sheath. These criteria, established in 1910 have remained intact, although the specified distinctions can now be evaluated with more detailed histochemical staining techniques [146]. These staining methods, subsequently, led to the study of electromechanical coupling [146, 159].
The atrioventricular node is located in the posteroseptal area, primarily on the right atrial side, at the apex of the region known as the triangle of Koch. This triangle is defined by a fibrous structure known as the Tendon of Todaro, the edge of the septal leaflet of the tricuspid valve and the edge of the mouth of the coronary sinus, which marks the base of the triangle. A second isthmus is present between the mouth of the coronary sinus and the septal leaflet of the tricuspid valve that is thought to support slow pathway contributions to the atrioventricular node. In the adult, the triangle measures 14–20 mm in its longest apex-to-base dimension. In children, as expected, the dimensions vary based on height, weight, body surface area age, and heart weight [163]. The atrioventricular node abuts the mitral valve annulus and tricuspid valve annulus with its posterior margin abutting the coronary sinus. Unlike the bundle of His, the atrioventricular node cannot be seen visually, nor does it generate a distinct, recordable signal during clinical electrophysiologic testing. The knowledge of its location is inferred during electrophysiologic testing in association with mapping techniques. The anterior portion or distal ends of the atrioventricular node blend with the bundle of His, which penetrates the central fibrous body. The atrioventricular node is thought to be a flattened, oblong structure with multiple extensions, some extending to the left atrium. The atrioventricular node is also thought to have extensions with a compact portion of the node existing more closely associated with the perimembranous portion of the ventricular septum. The atrioventricular node is usually supplied by an atrioventricular nodal artery, which arises from the right coronary artery in 90 % of cases and from the left circumflex artery in 10 % of the cases.
The bundle of His consists as an extension of the atrioventricular node. These extensions occur distal to the compact atrioventricular node. The bundle of His is characterized by fibers, which are organized in parallel channels or strands. These fibers are surrounded by a fibrous sheath more proximally and are, therefore, well insulated. The bundle of His penetrates the fibrous body and proceeds anteriorly descending towards the atrioventricular septum where it divides into the right and left bundle branches [164–166]. The compact atrioventricular node is thought to be buried inside the central fibrous body insulated by fibrous tissue continuing with extensions to the bundle of His and the bundle branches.
The right bundle is a relatively well defined and easily dissectible structure situated beneath the epicardium on the right side of the intraventricular septum. The right bundle branch proceeds along the free edge of the moderator band to the base of the anterior papillary muscles in the right ventricle and along the septal band to the apex of the right ventricle and to the “breakout point” on the anterior surface of the right ventricle [167].
The left bundle passes down the left side of the intraventricular septum and emerges below the posterior cusp of the aortic valve. In contrast to the right bundle, the left bundle breaks up almost immediately into a number of small fan-shaped branches, which proceed down the smooth aspect of the left side of the intraventricular septum. The bundle contains two major branches including an anterosuperior division and a posteroinferior division. The anterosuperior division is relatively long and thin whereas the posteroinferior division is relatively short and thick. The anterosuperior division is closer to the aortic valve whereas the posteroinferior division supplies the posterior and inferior aspect of the left ventricle [146].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


