Fig. 7.1
An anatomical plate of a human heart with the atria and great arteries removed showing the relationship between the four valves at the base of the heart. Note the fibrous connection between the leaflets of the mitral valve creating a double orifice valve
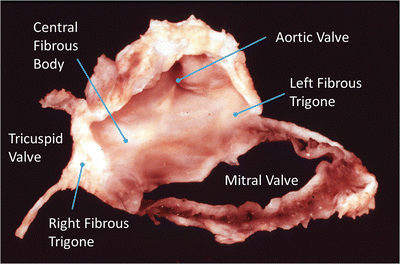
Fig. 7.2
Dissection of the cardiac skeleton showing the aortic valve (center), the mitral valve annulus (below right), and the fibrous sections of the tricuspid valve (to the left). The original image for this figure was kindly provided by Professor Robert H. Anderson. It was initially published in “Cardiac Anatomy” [3] and has been modified for this review. Professor Anderson retains the copyright of the initial image
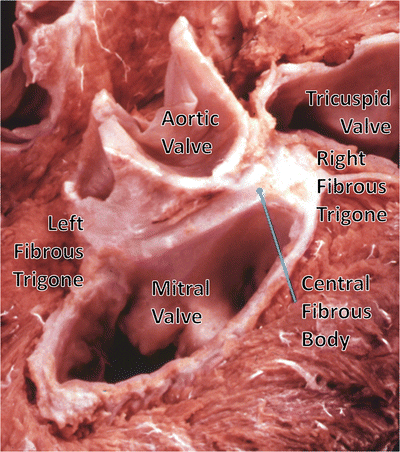
Fig. 7.3
Dissection of the cardiac skeleton with the atria and great vessels removed showing the coronet shape of the aortic annulus and the mitral valve. The original image for this figure was kindly provided by Professor Robert H. Anderson. It was initially published in “Cardiac Anatomy” [3] and has been modified for this review. Professor Anderson retains the copyright of the initial image
However, the extent of the skeleton is often greatly exaggerated. The so-called annular components of the atrioventricular valves then extend inferiorly and posteriorly from the central fibrous body and the left fibrous trigone, respectively (Fig. 7.3) [2]. It is the exception rather than the rule, however, for these fibrous cords to extend throughout the full circumference of the left and right atrioventricular junctions. The annuluses of the atrioventricular valves, as such, are better formed in the mitral as opposed to the tricuspid junction. In the mitral junction, it is normal to find segments of the valvar leaflets hinged from the fibroadipose tissue of the atrioventricular junction, rather than from a firm fibrous annulus [5]. In the tricuspid junction, the valvar leaflets are normally hinged from fibroadipose tissue [6]. The annuluses, as part of the atrioventricular junctions and rarely being complete fibrous rings, are highly dynamic and change dramatically in shape and size throughout the cardiac cycle from systole to diastole [1, 2]. It is also the fibroadipose tissue of the junctions that provides the greatest part of the insulation between the atrial and ventricular muscular masses, with the atrioventricular bundle of the conduction system being the only structure in the normal heart that crosses the insulating plane. The bundle penetrates through the atrioventricular component of the membranous septum.
The leaflets of the pulmonary valve have no direct fibrous support other than that provided by the valvar sinuses. The basal components of each leaflet are supported by the right ventricular infundibulum. It is this unique positioning of the pulmonary root away from the other valvar structures that makes possible its surgical removal during the Ross procedure , while the presence of the supporting skirt of infundibular musculature facilitates its use as an autograft to replace the aortic valve [7].
7.3 The Atrioventricular Valves
In the most basic anatomical sense, the atrioventricular valves are made up of three main components:
Valve leaflets attached to the respective annulus
Tendinous cords attaching the leaflets to the ventricular myocardium
Papillary muscles providing the anchoring points for the tendinous cords to the ventricular wall
The leaflets of the atrioventricular valves can be thought as forming a skirt that hangs from the annulus; leaflets are divided into a series of sections that constitute the distinct leaflets of each valve. Due to the extent of variations between individuals with regard to leaflet morphologies, there has been much debate relative to nomenclature on the number of leaflets of both the mitral and tricuspid valves [8–10]. Traditionally, the division of the leaflets has been determined by the presence of commissures which can be described as the peripheral attachment of a break in the skirt [1].
The leaflets themselves are attached to the ventricles via the sub-valvar apparatus of each valve. In general, each apparatus consists of the tendinous cords and the papillary muscle complexes of each valve. The tendinous cords are usually categorized by (1) those that support the free edges of the valves, (2) those that support the rough zones (the region between the free edge and each annulus), and/or (3) those that attach to the leaflets near to the annulus. Typically, the cords supporting the free edges of the leaflets are known as fan cords due to the presence of multiple fenestrations. Those that attach to the rough zone of the leaflets are distinguished by their larger size and are commonly defined as strut cords . Finally, those that attach near the annulus are known as basal cords . The strut cords are of specific importance, as they bear the highest mechanical loads during systole [11]. Furthermore, the number and distribution of the tendinous cords across a given valve are critical to its function; it is well documented that dysfunction of these structures can lead to prolapse of the valves [12–14]. In general, the cords attach to the heads of the papillary muscles, which themselves play an important role in the function of each valve by contracting during systole to cushion the valve closure and help prevent the valve from prolapsing into the atrium.
7.3.1 Atrioventricular Valve Function
During systole, when the ventricles are contracting, the sub-valvar apparatus of each valve prevents the leaflets from prolapsing into the atria and additionally aids in ventricular ejection by effectively drawing the apex of the ventricle toward the basal ring. Additionally, it has also been shown that the sub-valvar apparatus plays a crucial role during diastole, while the ventricle is filling, by moderating wall tensions and improving the efficiency of the ventricular myocardium [15, 16]. During systole in normal/healthy cardiac function, the valve leaflets, which bulge toward the atrium, can be considered to stay pressed together throughout the contraction and therefore do not prolapse. During diastole, when the ventricles are relaxing and the chambers are filling through the open atrioventricular valves, eddy currents that form behind the leaflets and tension in the sub-valvar apparatus keep the leaflets close together.
Figures 7.4 and 7.5 show image sequences obtained employing Visible Heart® methodologies, as described in Chap. 41. These sequences display the normal cardiac function of the mitral and tricuspid valves, respectively [17, 18]; the images were obtained from the atria (above the valve) and from the ventricular apexes (below the valve).
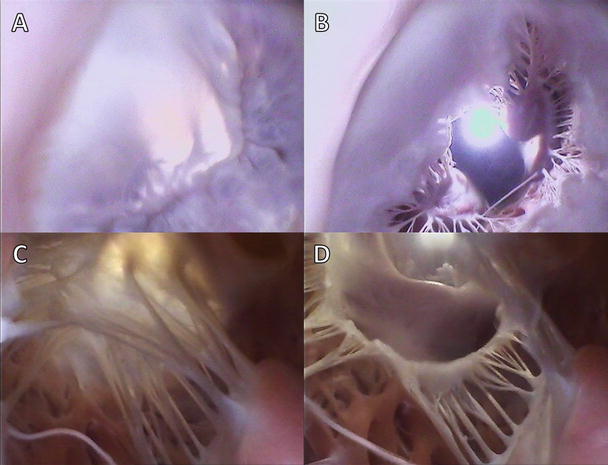
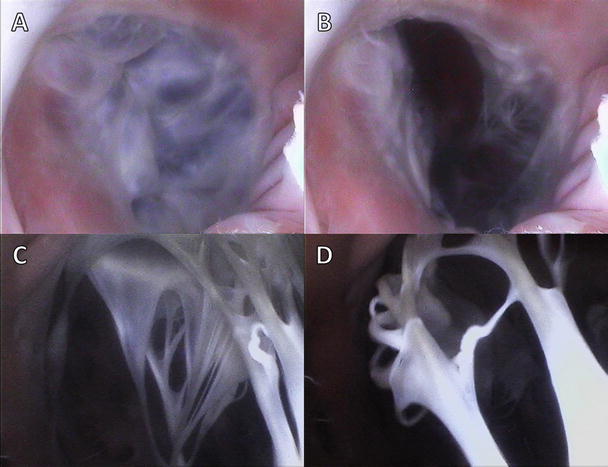

Fig. 7.4
Internal videoscopic images of the mitral valve from above (a, b) and below (c, d) during systole (a, c) and diastole (b, d) obtained employing Visible Heart® methodologies

Fig. 7.5
Internal videoscopic images of the tricuspid valve from above (a, b) and below (c, d) during systole (a, c) and diastole (b, d) obtained employing Visible Heart® methodologies
Dysfunction of the atrioventricular valves is usually characterized by one of two symptoms: (1) failure of a valve to successfully close or (2) failure of a valve to successfully open. Dysfunction of the valves during systole (i.e., failure of the valve to successfully close) is known as valvar incompetence and results in the regurgitation of blood back in a retrograde direction though the atrioventricular junction. Such dysfunction results in a decrease in cardiac output and also increases the pressure within the atria during systole (potentially causing atrial dilation and/or eventually atrial fibrillation). Dysfunction of the valves in diastole (failure of the valve to fully open and allow blood to fill the expanding ventricles) is termed stenosis . This decrease in effective orifice area of the open valve is often due to stiffening or calcification of the valve leaflets.
7.4 The Semilunar Valves
A healthy semilunar valve is composed of three valve leaflets, each attached to its respective sinus. These valves lie between the ventricular outflow tracts and the arterial trunks, the main arteries carrying blood away from the heart. This elegant structure is much simpler than that of the atrioventricular valves described previously, in that the semilunar valve leaflets do not require a tension apparatus to maintain competency. When closed, the three leaflets of each valve coapt along zones of apposition, or commissures, which are fibrous zones some distance from the free edge of the leaflets. At the center of the valve where all three leaflets coapt, a distinct fibrous nodule can be found. The valve leaflet margins are attached to the arterial wall in the shape of a half-moon, hence the semilunar moniker. Normally, the regions of the valves where the commissures meet the arterial wall are considerably higher than the seats of the leaflets, thereby giving the valve a crown-like shape. These three points, particularly in the aortic valve, are used to define the sinotubular junctions (Fig. 7.6). Although we have discussed the positioning of the valves in the heart by referring to their respective annuluses, many anatomists contest the idea that there are single defined annuluses for both the pulmonary and aortic valves [19]. Interestingly, there is a defined annulus where the respective arteries are attached to the ventricular outflow tract; however, due to the crown-like structure of the valve, the hemodynamic junction of the valves spans this annulus. This structural shape results in part of the arterial wall being considered a ventricular structure (in a hemodynamic sense) and, in turn, part of the ventricular wall an arterial structure. Just distal to the valves are the arterial sinuses that are represented by dilations of the artery positioned above each leaflet and additionally house the coronary artery ostium. The sinus also provides a recess for the valve leaflets to retract into, allowing for unrestricted flow from the ventricle to the artery. Finally, the virtual ring, upon which many annular measurements are based and which defines the basal plane of aortic valve, is defined by the three anatomical anchors at the nadir of each aortic leaflet [20]. These features are illustrated by the diagram in Fig. 7.6. The position and definition of the valve annulus is often contested by different medical specialists, and a recent questionnaire highlighted the current lack of consensus between physicians regarding the optimal means of describing the semilunar valve anatomy [21]. As such, it is important to be precise in the definition of exactly what is being measured when documenting the size and shape of the semilunar valve annuluses.
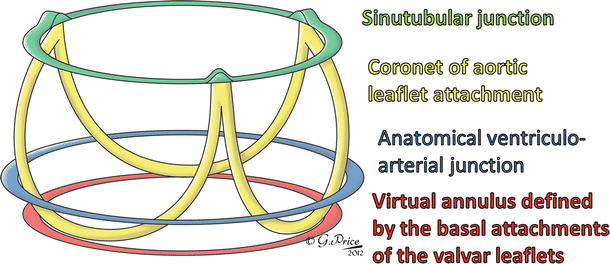

Fig. 7.6
Idealized three-dimensional arrangement of the semilunar valve (this diagram represents an aortic root). The model contains three circular rings with the leaflets suspended within the root in crown-like fashion. The cartoon is reproduced with kind permission of Professor Robert H. Anderson who retains the intellectual copyright in the original image. Special acknowledgment goes to Gemma Price as the artist [7]
7.4.1 The Functioning of the Semilunar Valves
When a semilunar valve is functioning correctly, the leaflets are pushed into the sinus during myocardial contraction (systole) to allow blood to leave the ventricles. As the myocardium relaxes and the pressure within the ventricle drops below the pressure distal to the valve in the arterial system (the aorta or pulmonary artery), the valve snaps shut. This usually happens soon after ventricular systole but before the heart has completely relaxed, so that during diastole, when the chambers are filling through the atrioventricular valves, the leaflets of the semilunar valves remain tightly closed. A positive pressure difference between the aorta and the coronary sinus, which lies within the right atrium, allows for the flow of blood through the coronary vasculature. Thus, it should be noted that the heart muscle is perfused with blood when the semilunar valves are closed and the cardiac myocytes are relaxing.
Figures 7.7 and 7.8 show image sequences of the functional movements of the pulmonary and aortic valves, respectively; these images were obtained from reanimated human hearts employing Visible Heart® methodologies [17, 18]. The images include views of semilunar valves from above (i.e., from videoscopes within the pulmonary artery and the aorta) and from below (with videoscopes within the right and left ventricular outflow tracts).
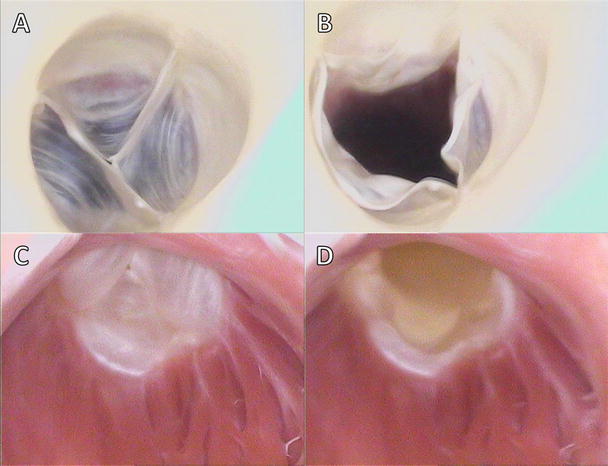
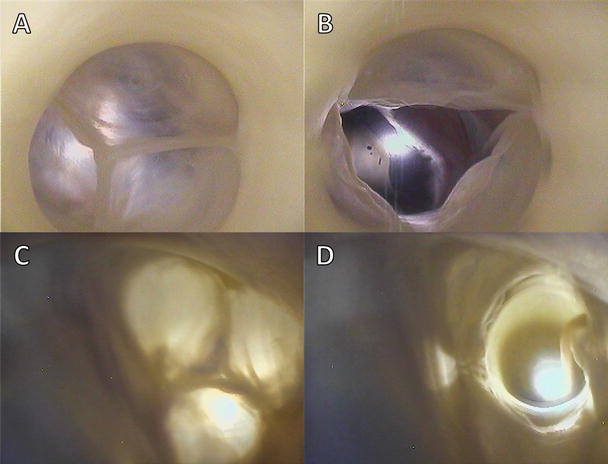

Fig. 7.7
Internal videoscopic images of the pulmonary valve from above (a, b) and below (c, d) during systole (a, c) and diastole (b, d) obtained employing Visible Heart® methodologies

Fig. 7.8
Internal videoscopic images of the aortic valve from above (a, b) and below (c, d) during systole (a, c) and diastole (b, d) obtained employing Visible Heart® methodologies
In general, dysfunction of the semilunar valves is usually characterized by one of two symptoms: failure of the valves to successfully close or failure of the valves to successfully open. Dysfunction of the valves during systole, i.e., failure of the valve to successfully open, is defined as stenosis of the valve. This pathology is characterized by reduction in the effective orifice area of the valve (the size of the opening that allows blood to pass), which in turn forces the ventricles to work harder to move blood to the body or lungs. Dysfunction of the semilunar valves during diastole, when the ventricles are relaxing, results in regurgitation; this occurs when blood is allowed back into the ventricle from the arterial system, overloading the ventricles and potentially causing chronic heart failure.
7.5 Valve Histologies
Interestingly, the atrioventricular valves share very similar leaflet histology. The atrial sides of the leaflets consist of spongy tissue (lamina spongiosa) comprised of fibrocytes, histiocytes, and collagen fibers [22]. It is these collagen fibers that are considered to supply the mechanical strength required of the atrioventricular valves. The ventricular sides consist of fibrous tissue (lamina fibrosa), and both these layers are surrounded by endothelial cells. Additionally, the valve leaflets have been shown to incorporate both primary sensory and autonomic innervation. In general, it is considered that the anterior leaflet of the mitral valve has twice the innervation of the posterior leaflet [23]. These nerves are typically situated in the lamina spongiosa and extend over the proximal and medial portions of the leaflet [22]. Fibroblasts [24], smooth muscle cells [25, 26], and myocardial cells [27] are also commonly located within the leaflet tissue.
Cells within the leaflets have been shown to elicit two types of contractile activity : (1) a brief contraction or twitch at the beginning of each heartbeat (reflecting contraction of myocytes in the leaflet in communication with, and excited by, atrial muscle) which has relaxed by mid-systole and whose contractile activity is eliminated with β-receptor blockade, and (2) sustained tonic contractions (or tone) during isovolumic relaxation, which has been shown to be insensitive to β-blockade, but doubled by stimulation of the neurally rich region of aortic-mitral continuity [28]. These contractile activities within the leaflets are hypothesized to aid in the maintenance of anterior leaflet shape. This, in turn, could help prevent mechanical shock to the leaflets upon valve closure and also aid in optimizing the leaflet shape for funneling blood into the left ventricular outflow tract [28].
The tendinous cords are composed of a collagen core, surrounded by elastin fibers interwoven in layers of loose collagen. Similar to the valve leaflets, they also have an outer layer of endothelial cells, but it is the collagen cores that support the greatest degree of mechanical load during systole and allow for the wavy configuration during diastole. The elastin fibers are normally arranged in parallel fashion relative to the collagen fibers, and as the cords are stretched during systole, the elastin fibers are also stretched, straightening the collagen. It is hypothesized that it is this composite configuration of elastin and collagen that provides a smooth mechanism for the transmission of cordal forces from the leaflets to the papillary muscles. Additionally, during diastole, the stretched elastin fibers likely help to restore the wavy configuration of the primary collagen cores. The relative amount of collagen and elastin within the given chordae varies according to their relative types, as does the relative amount of contained DNA and their degree of vascularization. Normally the vascularization of the tendinous cords is located between their collagen cores and the elastin fibers and is further considered to supply nutrients to the leaflets. It has been reported that a higher DNA content within both the anterior and posterior marginal chordae relates to inherently higher rates of collagen syntheses in order to prevent mechanical deterioration compared with other types of chordae [13].
The papillary muscles can be considered part of the ventricular myocardium and hence are composed of aggregated myocytes. The cells exhibit complex junctions, called intercalated discs , allowing multiple cells to form long cellular networks. Within the papillary muscles, these muscle fibers run parallel to each other along the length of the muscle to increase contractile force and efficiency. The papillary muscles are extensively innervated and have complex vascular systems in order to maintain coordinated contractions with the continuum of the ventricular myocardium [29].
It was Gross who first drew attention to the specific histological structures of the arterial valves, his account then being endorsed by others such as Misfeld and colleagues [22, 30]. Each leaflet of the semilunar valve was described to have a fibrous core, or fibrosa , with an endothelial lining containing delicate sheets of elastin on its arterial and ventricular aspects. This so-called fibrous “backbone” is represented by a dense collagenous layer, which gives way to a much looser structure, or spongiosa , toward the ventricular aspects of the leaflet cusps. The zone of apposition of the leaflets consists of an abrupt thickening of the fibrous layer made up of closely packed vertically directed fibers and builds at the central portion of the free edge, creating a node termed the nodulus Arantii [22, 30]. Figure 7.9 displays a cross section of an aortic valve leaflet displaying the varying tissue types [31].
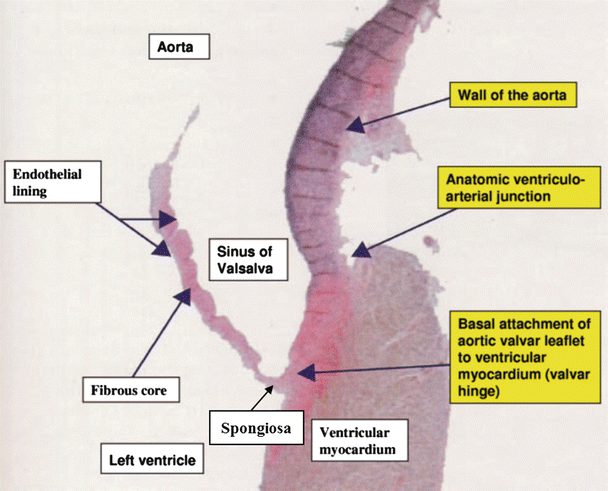

Fig. 7.9
Histologic features of the aortic valvar complex showing the anatomic ventriculoarterial junction. Also note that the basal attachment of the aortic valvar leaflets to the ventricular myocardium is proximal relative to the anatomic junction. Image is reproduced with permission from Piazza N et al. (2008) [31]
7.6 The Mitral Valve
The left atrioventricular valve, or mitral valve, named by Andreas Vesalius due to its structural resemblance to the cardinal’s mitre, is situated in the left atrioventricular junction and modulates the flow of blood between the left atrium and ventricle. Commonly, the valve consists of an annulus, two leaflets, two papillary muscle complexes, and two sets of tendinous cords, as seen in Fig. 7.10.
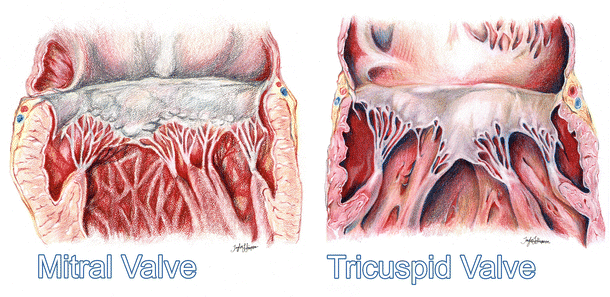

Fig. 7.10
An artist’s rendition of the healthy mitral and tricuspid valves clearly showing the annuluses, leaflets, tendinous cords, and papillary muscles
In 1976 Carpentier described the mitral valve as consisting of two apposing leaflets—a posterior leaflet with three scallops and an anterior leaflet with one scallop. Each region of the leaflets is designated an alphanumerical label to distinguish it from the rest of the valve (Fig. 7.11) [32]. However, when one considers these structures relative to the landmarks of the body (i.e., in an attitudinally correct nomenclature), the leaflets are located in posteroinferior and anterosuperior positions. Confusion regarding positional nomenclature can be avoided when adopting the more traditional approach suggested by Vesalius for distinguishing between the leaflets and recognizing that they are aortic and mural in their locations [33]; in this chapter, we will use such nomenclature. The junctions of the two leaflets are commonly referred to as the anterolateral and the posteromedial commissures ; however, they are more accurately described as superior and inferior. The line of apposition of the leaflets during valve closure is known as the fibrous ridge . The simplicity and practicality of Carpentier’s anatomic description of the mitral leaflets led to its widespread use after being introduced in 1976 [32]; yet, while this description depicts a majority of mitral valve anatomies, there can be wide variability in the number of scallops within each leaflet and their relative positions [34].
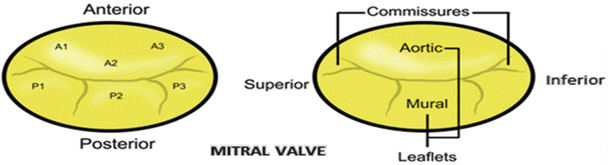

Fig. 7.11
Nomenclature of the mitral valve leaflets . The left diagram shows Carpentier’s 1976 nomenclature, while the right depicts the modern attitudinally correct nomenclatures
In general, the aortic leaflet is found to be attached to approximately one-third of the annulus circumference and is supported by the aorto-mitral fibrous continuity, which terminates in the left and right fibrous trigones (Fig. 7.12). The mural leaflet is attached to the remaining two-thirds of the annulus and also to the fibrous extensions that continue from the trigones around the mitral valve. However, the lengths of these extensions can be highly variable. Furthermore, a fibrous-fatty tissue surrounds the valve in areas where the cardiac skeleton is not present. The mitral annulus is a highly dynamic feature of the heart, changing dramatically in shape and size throughout the cardiac cycle. It is often described as being saddle-shaped with the highest point of the saddle, the saddlehorn, being found at the midpoint of the area of aortic-to-mitral valvar continuity [35] (Figs. 7.4 and 7.13). Both Delgado and Veronisi and their colleagues reported a series of annular dimensions that were recorded using echocardiography in healthy patients; these data are summarized in Table 7.1 [36–38].
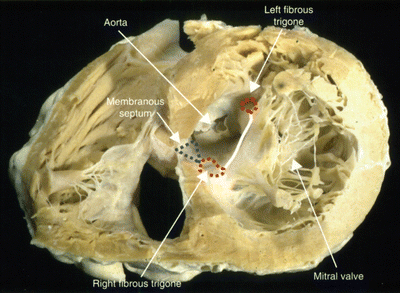
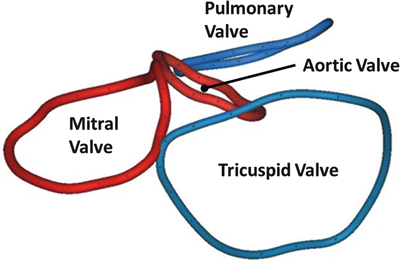

Fig. 7.12
Trigones and the aorto-mitral fibrous continuity within a sectioned human heart. In this case, the cardiac skeleton is being viewed from the apex of the heart. The anterior cardiac surface appears in the upper part of this image, whereas the posterior surface is below. Image is reproduced with permission from Anderson RH et al. (2006) [33]

Fig. 7.13
3D reconstruction of the annuluses of the mitral (red) and tricuspid (blue) valves in Mimics ® (Materialise, Leuven, Belgium) from CT scans of a human heart in vivo. The image also shows the location of the virtual rings formed by joining together the basal attachments of the leaflets of the aortic and pulmonary valves
Measured anatomical feature | Data | Sample size |
|---|---|---|
9.12 ± 1.71 cm2 9.49 ± 1.25 cm2 | n = 84 n = 13 | |
2.38 ± 0.40 cm 3.00 ± 0.45 cm | n = 84 n = 13 | |
4.10 ± 0.48 cm 3.42 ± 0.40 cm | n = 84 n = 13 | |
Annulus height during systole [38] | 8.1 ± 1.7 mm | n = 24 |
In general, the sub-valvar apparatus of the mitral valve consists of two adjacent papillary muscle complexes—the superoposterior (anterior or APM) and the inferoanterior (posterior or PPM)—with their attached tendinous cords which, in turn, insert onto the ventricular surfaces of each of the two valve leaflets [33]. In other words, the superoposterior papillary muscle complex is not solely associated with the aortic leaflet, but rather both the leaflets; likewise, the inferoanterior papillary muscle complex is not solely associated with the mural leaflet. It is important to note that the morphologies of the papillary muscle complexes are highly variable [36]. Some have proposed a complicated alphanumeric classification to account for the number of heads within each muscle and the number of attachments with the ventricular walls [39]. Even this complex code can be deemed as an oversimplification, as both papillary complexes can exhibit enormous anatomic variation [40]. For example, Fig. 7.14 displays images of the sub-valvar apparatus of the mitral valve taken from human hearts in the Visible Heart® library [40].
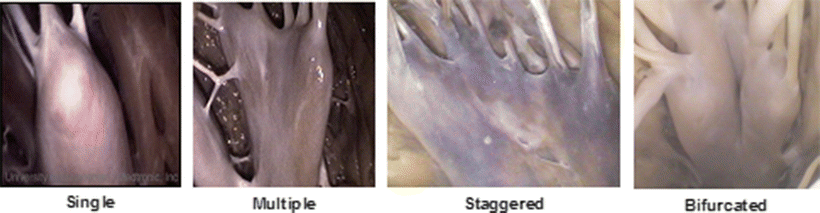

Fig. 7.14
Several representative examples of the enormous variation in anatomy with regard to the papillary muscle heads associated with the mitral sub-valvar apparatus from four different human hearts taken using Visible Heart® methodologies [40]
The tendinous cords are typically classified by their number and length and quantified by one of two measurement techniques—tethering length and insertion length. Tethering length is defined as the distance from the papillary head to the saddlehorn of the mitral annulus. Insertion length is defined as the length of the cords from their origin at the papillary head to their insertion into the leaflet tissue. Anatomical dimensions obtained from patients with no reported mitral regurgitation or other valvar pathologies were reported by Sonne et al. and Lam et al. and are summarized in Table 7.2 [41, 42]. Yet, it should be emphasized that, as with other anatomical studies, these data do not account for all anatomical variations. For example, it has also been reported that the cordal attachments to the mural leaflet may extend simply from the ventricular myocardium to the leaflet without a papillary muscle attachment. Furthermore, it is well known that the tendinous cords themselves may elicit highly variably anatomies, and various subpopulations of chordae have been classified by both function [43] and type [44]. Figure 7.15 shows examples of these identified variations in the types of chordae, including posterior marginal chordae, commissural chordae, anterior strut chordae, anterior marginal chordae, basal posterior chordae, and posterior intermediate chordae.
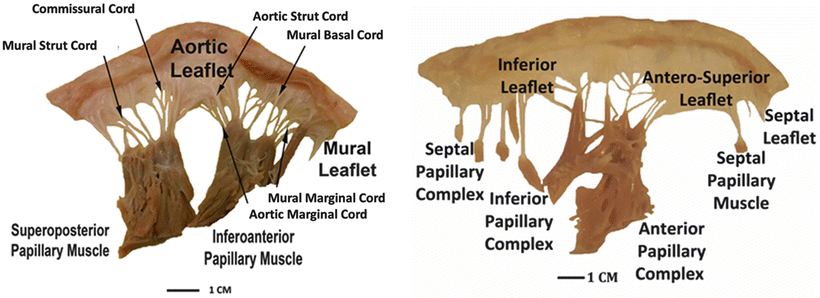

Fig. 7.15
Dissections of a human mitral valve (left) and tricuspid valve (right), each labeled with attitudinally correct nomenclature. Note the dramatic differences between the two valves, including their respective sub-valvar apparatuses
Measured anatomical feature | Data | Sample size |
|---|---|---|
APM tethering length in systole [41] | 3.54 ± 0.82 cm | n = 120 |
PPM tethering length in systole [41] | 3.76 ± 0.78 cm | n = 120 |
Anterior leaflet insertion length [42] | 1.81 ± 0.49 cm | n = 50 |
Posterior leaflet insertion length [42] | 1.18 ± 0.26 cm | n = 50 |
Ratio of chordal origins to insertions [43] | 1:5 | n = 18 |
7.7 The Tricuspid Valve
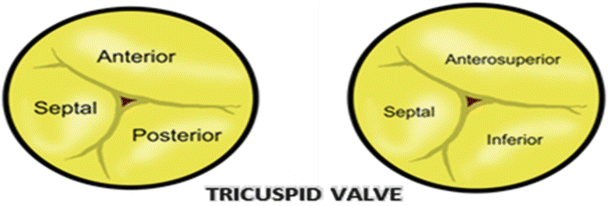
The right atrioventricular valve, or tricuspid valve , is situated within the right atrioventricular junction and modulates the flow of blood between the right atrium and right ventricle. This valve is typically defined by three leaflets suspended from the muscular atrioventricular junction and connected to the ventricular wall via three distinct papillary muscle complexes (as seen in Fig. 7.10). When defined using attitudinally correct nomenclature, these leaflets are located in septal, anterosuperior (traditionally anterior), and inferior (traditionally posterior) positions (Figs. 7.16 and 7.17) [45].
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


