Chapter 34 Descending Thoracic and Thoracoabdominal Aortic Aneurysms
General Principles and Open Surgical Repair
Although descending thoracic and thoracoabdominal aortic aneurysms are not as common as infrarenal aortic aneurysms, repair is problematic because of the involvement of visceral arteries and vital lumbar arteries supplying the spinal cord. The classification systems for descending thoracic and thoracoabdominal aortic aneurysms were devised because different aneurysm extents have different levels of risk (Figure 34-1). Since its inception, thoracoabdominal aortic aneurysm repair has been plagued with complications stemming from spinal, mesenteric, and renal ischemia. Adjunctive surgical techniques led to significant reductions in morbidity, especially because of spinal ischemia.
The first reported repairs of descending thoracic aortic aneurysms were published in the early 1950s.1,2 The first to successfully perform a thoracoabdominal aortic aneurysm repair was Samuel Etheredge and colleagues in 1955 in Oakland, California.3 One year later, Michael DeBakey and colleagues performed a true thoracoabdominal repair using a homograft. Subsequently, the same group devised an ingenious method of using a Dacron tube graft from the descending thoracic aorta to the infrarenal aorta, and sequentially bypassing the celiac axis, superior mesenteric artery, and both renal arteries. This technique both decreased the load on the heart and the ischemic time to the bowels and the kidneys. Dr. Crawford’s repair of thoracoabdominal aortic aneurysm incorporated three principles of vascular surgery: the Matas inclusion technique,4 whereby the aneurysm sac is opened but not resected; the reattachment of the intercostal arteries to the graft (as described in an animal model by Spencer in 19505); and the reattachment of the small arteries into a large artery attributed to Carrell and Guthrie in 1906. Crawford accrued 28 cases by 1973 and published his results of a large group of aneurysm cases treated for the first time with the same clamp-and-sew technique.6 This technique depended on expeditious repair with short aortic clamp times because the spine and visceral vessels suffered ischemia during repair. Several adjunctive methods for spinal cord and visceral protection later emerged, including distal aortic perfusion,7–9 cerebrospinal fluid (CSF) drainage,10 and systemic or regional profound hypothermia.11
Natural History
The exact prevalence of descending thoracic and thoracoabdominal aortic aneurysms can only be inferred. The incidence of thoracic aneurysms is increasing likely because of better detection with echocardiography and computed tomography. One population-based study from Olmsted County, Minnesota, estimated the incidence of thoracic aneurysms at 10.4 per 100,000 people.12 The most common thoracic aneurysm occurs in the ascending aorta, but descending thoracic aortic aneurysms account for 45%, whereas thoracoabdominal aortic aneurysms account for approximately 5% of all thoracic aortic aneurysms.13
Nearly 13,000 people die annually from thoracic aortic aneurysm, making it the nineteenth leading cause of death in the United States.14 This rate reflects a dramatic increase to 13.5 and 33.4 per 100,000 in age groups of 65 to 74 and 75 to 84 years.15 Overall, aortic aneurysm and dissection accounted for 4.3 deaths per 100,000 people in the United States in 2007. Death from thoracic aortic aneurysm is primarily due to rupture. The rate of rupture increases with aneurysm expansion.16,17 The natural history of thoracic aortic aneurysm is expansion at a median 0.1 to 0.3 cm/year.16,18 After 5 cm, growth and rupture can occur unpredictably. In the Olmsted County study, no ruptures occurred in thoracic aneurysms less than 4 cm. The 5-year rupture risk increased to 16% for aneurysms less than 6 cm and was greater than 30% for aneurysms larger than 6 cm.12 After rupture, the mortality rate is greater than 90% in the first 24 hours.19 Most patients die before reaching the emergency room.
Pathology
An aneurysm is a localized or diffuse dilatation greater than 50% of the reference diameter. Most aneurysms are due to medial degeneration and appear to occur sporadically, but genetic factors are increasingly recognized. Marfan syndrome is an inherited connective tissue disorder caused by a mutation of fibrillin-1 protein that is encoded in the FBN1 gene. Other syndromes that predispose individuals to abdominal aortic aneurysms include Loeys-Dietz syndrome, variants of Ehlers-Danlos syndrome, and Turner syndrome. Mutations in α-actin, such as ACTA2, highlight the importance of vascular smooth muscle function in aortic aneurysm and dissection.20 In addition, other genes contributing to thoracic aortic disease include MYH11, TGFBR, and SMAD3.21 The recognition of genetic variants in the development of aortic aneurysm will surely increase because many patients report multiple family members with thoracic aortic disease despite a negative genetic workup.
Like atherosclerosis, aortic aneurysm pathophysiology includes a robust inflammatory component. However, unlike atherosclerosis, which primarily affects the intima and causes occlusive disease, the inflammation in aneurysms occurs in the media and adventitia. Histology of degenerative aortic aneurysms is characterized by thinning of the media with distraction of the smooth muscle and elastic tissue (elastin). There is infiltration of inflammatory cells, including mast cells with neovascularization.22 There is also activation of proteolytic enzymes and their inhibitors within the aortic wall.23
A small percentage of aneurysms are related to infection or so-called mycotic infection, or to trauma, such as pseudoaneurysm. Bacteria or septic emboli may seed the atherosclerotic or ulcerated aortic wall. Infection may also spread from an adjacent empyema or lymph nodes. Organisms associated with aortic infection include Salmonella species, Haemophilus influenzae, Staphylococcus species, Mycobacterium tuberculosis, and Treponema pallidum. These aneurysms are usually saccular and carry a high risk of rupture. Traumatic injuries of the thoracic aorta are also prone to rupture and should be repaired unless the injury is confined to the intima.24–27
Clinical Manifestation
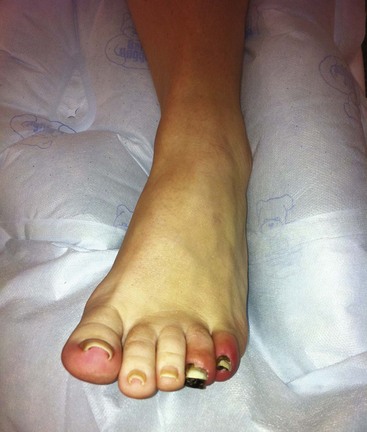
Many thoracic and thoracoabdominal aneurysms are discovered incidentally and most often develop without symptoms. Indications for surgical intervention depend on the size of the aneurysm and the presence or absence of symptoms. Symptoms are usually due to sudden expansion of the aneurysm, which can cause a vague pain in the back or sometimes a sharp pain that may denote the presence of rupture or impending rupture. Other symptoms are related to pressure on adjacent structures, such as pressure on the bronchus that can cause respiratory distress or pressure on the recurrent laryngeal nerve causing vocal hoarseness (Figure 34-2). Pressure on the esophagus can cause difficulty swallowing. On rare occasions, paraplegia and paraparesis can be caused by occlusion of critical intercostal arteries. There is also a risk of distal embolization causing “blue toe” syndrome (Figure 34-3).
Certain manifestations of rupture in 10% to 20% of thoracoabdominal aortic aneurysms include the sudden onset of severe or sharp pain radiating to the back causing a drop in hemoglobin and blood pressure. In a review of more than 100 ruptured descending thoracic and thoracoabdominal aortic aneurysms, the most common site of rupture was the pleural cavity.28 Other sites of rupture occurred into the lung, esophagus, mediastinum, retroperitoneum, and peritoneal cavity. Thus, in addition to back, chest, or abdominal pain, patients may also exhibit with hemoptysis or hematemesis.
Diagnosis
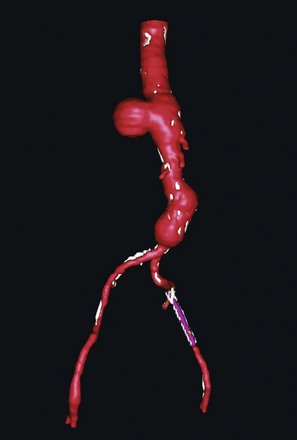
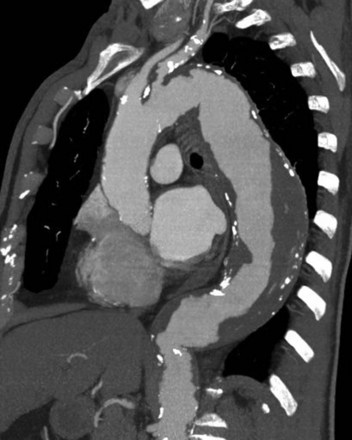
In the past, the definitive diagnostic tool was the aortogram; however, with the emergence of axial imaging, computed tomography (CT) or magnetic resonance angiography (MRA) are the modalities of choice. CT allows for visualization of the aortic wall, the aneurysm size, the presence or absence of clot formation, intercostal arteries, and effects on the surrounding tissues. The disadvantage of CT is the effect of ionizing radiation and the contrast media, which can exacerbate poor renal function. Three-dimensional reconstruction of axial imaging provides an additional view of thoracoabdominal aortic aneurysms (Figure 34-4). MRA is widely available and has the advantage of not using ionizing radiation, but it takes longer, provokes claustrophobia, and is contraindicated for patients with cerebral aneurysm clips, certain mechanical valves, or other internal metallic hardware. MRA is used less frequently because of these limitations.
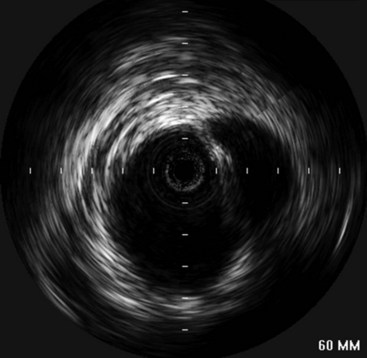
Intravascular ultrasound (IVUS) is emerging as a useful adjunct, especially when there is diagnostic uncertainty such as in traumatic aortic injury (Figure 34-5). This modality allows for high-resolution imaging of the aortic wall and can often distinguish intimal lesions better than axial imaging.29 The drawback is that it requires invasive intraarterial access, as well as the additional cost of the catheter and computerized ultrasound equipment.
Thoracic Aneurysm Classification
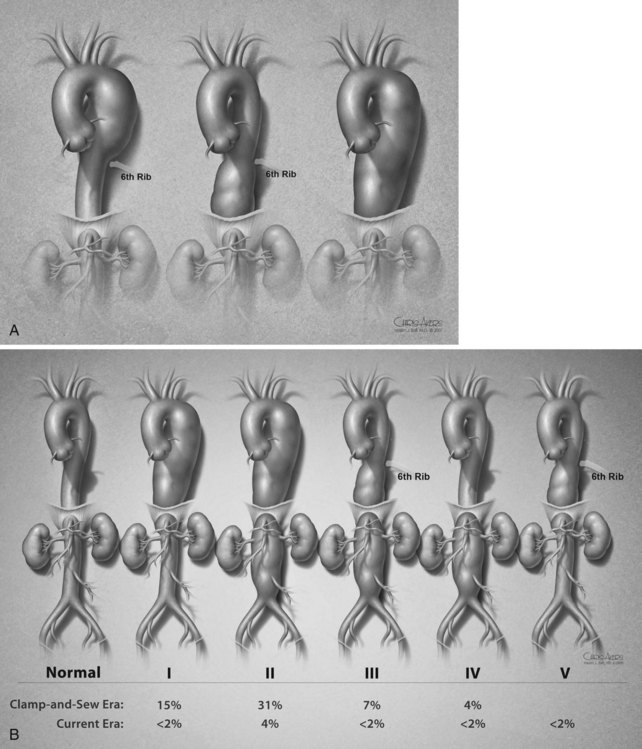
Descending thoracic aortic aneurysms are divided into three extents (see Figure 34-1A).30 Extent A designates an aortic aneurysm from the distal origin of the left subclavian artery to the level of the sixth thoracic intercostal space (T6). Extent B is from T6 to the twelfth thoracic intercostal space (T12). Extent C denotes the entire descending thoracic aorta from the left subclavian artery to T12.
Thoracoabdominal aortic aneurysms are classified in five categories (see Figure 34-1B). Extent I is from the left subclavian to above the most proximal renal artery. Extent II is from the left subclavian artery to below the renal arteries. Extent III is from the sixth intercostal space to below the renal arteries. Extent IV is from T12 to below the renal arteries. Extent V, which was introduced in the last 2 decades, is from T6 to just above the renal arteries. The importance of the classification scheme is that it correlated with the incidence of neurologic deficits and mortality especially when using the clamp-and-sew technique.
Preoperative Evaluation
Preoperative evaluation of patients for elective thoracoabdominal aortic aneurysm should include a thorough history, physical examination, and determination of the presence of cardiovascular, pulmonary, and renal diseases. Pulmonary function testing is obtained in all patients, especially those with a history of pulmonary disease. However, there is still controversy regaining optimal preoperative cardiac workup for noncardiac vascular disease, despite published practice guidelines.31,32 Preoperative left ventricular ejection fraction was the strongest cardiac predictor of mortality,33 and echocardiography is obtained in all patients to assist in stratifying risk and to determine the need for additional cardiac testing. Treatment of coronary artery disease can be treated with medical therapy, coronary artery angioplasty, or coronary artery bypass before thoracoabdominal repair. It is unclear whether preoperative coronary artery or carotid artery revascularization are beneficial.34,35
Placement of stents during percutaneous coronary interventions requires antiplatelet medications to prevent in-stent thrombosis.36 However, administering these can present a problem because newer antiplatelet medications are more potent and can predispose patients to bleeding after major aortic surgery.37 Cessation of antiplatelet medications with drug-eluting stents is associated with greater than threefold the thrombosis rate compared with bare metal stents. In addition, more than one in four people require noncardiac surgery within 5 years after stent implantation.38 Current guidelines suggest waiting 2 to 3 months after bare metal coronary stenting and 1 year after drug-eluting stent placement before holding antiplatelet medications for elective surgery.39 However, the thrombosis risk for drug-eluting stents persists even after 12 months from implantation.38,40,41
Renal function should be evaluated with estimation of the glomerular filtration rate (GFR). Normal values are greater than 90 mL/min per 1.73 m2. GFR is easy to estimate by several methods (e.g., Cockcroft-Gault, Modification of Diet in Renal Disease, or Chronic Kidney Disease Epidemiology Collaboration formulas) and is a better predictor of risk than serum creatinine.42 Many formulas are available in smart phone applications and can be calculated in seconds.
Axial imaging of the entire chest, abdomen, and pelvis is mandatory to determine the extent of the aneurysm as well as synchronous aneurysms (Figure 34-6). Use of intravenous contrast is helpful in determining the presence of thrombus and atheromatous disease in the aortic wall (see Figure 34-6). Echocardiography is also helpful to evaluate for atheromatous disease in the thoracic aorta.
Surgical Technique
General endotracheal anesthesia is induced with a double-lumen tube for selective single-lung ventilation. A pulmonary artery catheter and neuromonitoring leads for motor-evoked and somatosensory evoked potentials are placed. The patient is positioned in the right lateral decubitus position for placement of the CSF drain. After securing the drain, the hips are tilted slightly posteriorly to gain access to the left femoral artery (Figure 34-7). The shoulders are positioned perpendicular to the table. CSF pressure is kept at 10 mm Hg or less. This pressure is maintained intraoperatively and for 3 days postoperatively.
A thoracoabdominal incision is tailored to fit the extent of the aneurysm. In descending thoracic aortic aneurysm (DTAA) and thoracoabdominal aortic aneurysm (TAAA) extent I and V, a smaller modified thoracoabdominal incision is preferred (see Figure 34-7). The full thoracoabdominal incision begins posterior to the tip of the scapula and proceeds medially along T6. The incision is extended inferiorly at the midline toward the umbilicus. Next, the latissimus dorsi is divided and the serratus anterior muscle is mobilized. After the left lung is deflated, the left chest is entered. The sixth rib is resected or cut posteriorly and left in place, and a self-retaining retractor is used to aid in exposure.
Cannulas are placed for distal aortic perfusion. A purse-string suture is placed in the left inferior pulmonary vein, and the vein is incised. Intravenous heparin is given at 1 mg/kg, and the left inferior pulmonary vein is cannulated with the catheter tip directed toward the left atrium. The catheter is attached to a centrifugal pump with an in-line heat exchanger. The left common femoral artery is exposed and a graft is sewn in an end-to-side fashion to provide inflow. Instead of directly cannulating the femoral artery, a side-arm graft is used to preserve renal function and reduce extremity muscle ischemia.43,44
A segmental clamp is applied to the proximal descending thoracic aorta and the mid-thoracic aorta before transecting the aorta. In general, the inclusion technique at the proximal anastomosis is no longer used. The proximal aorta is divided completely and separated from the underlying esophagus to mitigate the risk of graft-esophageal fistula (Figure 34-8). A collagen or gelatin-impregnated woven Dacron graft is sutured end-to-end with 2-0 or 3-0 polypropylene sutures. In cases of acute dissection, 4-0 polypropylene sutures can be used. After completion of the proximal anastomosis, the clamp is moved distally to the celiac axis. The remaining thoracic aorta is opened, and upper intercostal arteries are ligated. Lower thoracic intercostal arteries are temporarily occluded with balloon-tipped catheters.

FIGURE 34-8 Separation of the aorta from the underlying esophagus in preparation for the proximal anastomosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree