CLINICAL PRESENTATION
d-Transposition of the great arteries (d-TGA) is one of the two most common forms of cyanotic congenital heart disease, with tetralogy of Fallot having comparable incidence. In population-based series, the incidence of d-TGA is 20 to 22 per 100,000 live births. Typically, neonates with d-TGA present in the first day or two of life with cyanosis without significant respiratory distress. Frequently no cardiac murmur is present, particularly in the absence of a ventricular septal defect (VSD). The electrocardiogram and chest radiograph are frequently normal. If a VSD or outflow tract obstruction is present, a murmur characteristic of these associated lesions may be present.
Maintenance of a compensated clinical state is dependent on adequate mixing between the pulmonary and systemic circuits. Neonates with an intact atrial septum or very restrictive patent foramen ovale can present with profound cyanosis and findings consistent with low cardiac output in the delivery room. Those whose intercirculatory mixing is initially augmented by a patent ductus arteriosus (PDA) can develop worsening cyanosis and low cardiac output as the ductus constricts. Uncommonly, neonates with d-TGA who have good intercirculatory mixing escape notice in the first few days of life and may present later with signs of heart failure, typically accompanied by a murmur originating from either excessive flow across the left ventricular outflow tract or across a VSD.
Diagnosis by fetal echocardiography is another mode of presentation, generally prompted by abnormal findings on a routine obstetric screening ultrasound. Prenatal detection of d-TGA by echocardiography requires examination of the outflow tracts, because examining only the number and size of cardiac chambers will not detect this anomaly (Figs. 16.1 to 16.4; Video 16.1). The frequency of antenatal diagnosis of d-TGA varies considerably, ranging from 20%–50% in recent publications.
ANATOMY AND PHYSIOLOGY
In d-TGA, the atrial situs, atrioventricular alignments, and ventricular looping are all normal. TGA is present, meaning that the aorta arises from the right ventricle while the pulmonary artery arises from the left ventricle (Fig. 16.5). The designation d- refers to the relative positions of the aortic and pulmonary valves; d- (dextro-) indicates that the aortic valve is rightward of (and typically anterior to) the pulmonary valve, in accordance with the convention established by Van Praagh. There may be other associated malformations, most commonly the presence of a VSD, which occurs in approximately 35% to 40% of cases and can be of various anatomical types.
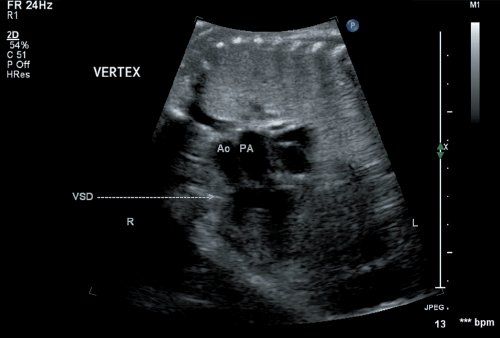
Figure 16.1. Fetal echocardiogram performed at 20 weeks’ gestation. Imaging from the four-chamber view (arrow), directed anteriorly, demonstrates the parallel nature of the great arteries. The great vessels appear symmetrical with a large outlet ventricular septal defect positioned directly below the semilunar valves. Ao, aorta; PA, pulmonary artery.
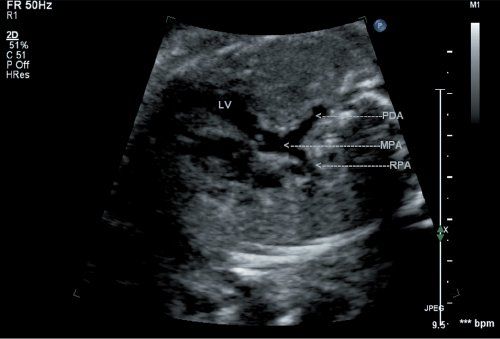
Figure 16.2. Fetal d-transposition of the great arteries (d-TGA) at 20 weeks’ gestation demonstrates a well-developed pulmonary artery arising posteriorly from the left ventricular chamber (arrow). The patent ductus arteriosus is directed posteriorly toward the spine, and the right pulmonary artery branch is also seen. LV, left ventricle; PDA, patent ductus arteriosus; MPA, main pulmonary artery; RPA, right pulmonary artery.
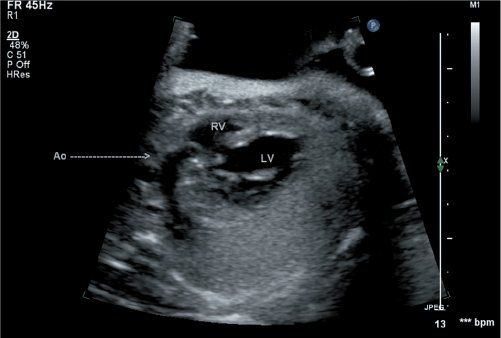
Figure 16.3. Scanning anteriorly in this fetus at 20 weeks gestation, the anterior aorta is seen to arise from the right ventricle (arrow). Two head-and-neck vessels are seen originating from the transverse aortic arch, confirming this as the true aortic arch. A ventricular septal defect is noted in the region of the outlet septum. RV, right ventricle; LV, left ventricle.
The physiologic consequence of these anatomic relationships is that two parallel circulations are established. Systemic venous blood returns to the right atrium and from there passes to the right ventricle and aorta and back to the systemic arterial bed without being oxygenated. Pulmonary venous blood returns to the left atrium and thereafter passes to the left ventricle, pulmonary artery, and pulmonary arterial bed without any opportunity to deliver its oxygen cargo. This arrangement is only compatible with life if some intercirculatory mixing (bidirectional shunting) is present. Mixing can occur via an interatrial communication (patent foramen ovale or true atrial septal defect), VSD, or PDA, but is most effective across an atrial septal defect.
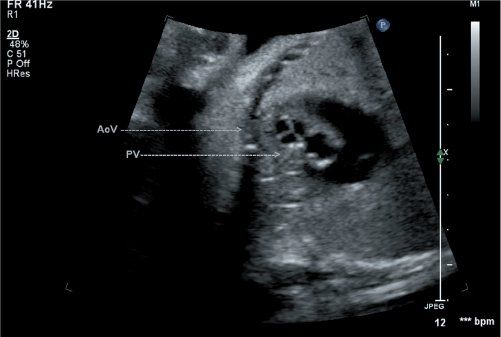
Figure 16.4. Short-axis view of the semilunar valves in a fetus with d-TGA. The aortic valve is seen to be trileaflet and positioned anterior (arrows) and rightward relative to the pulmonary valve. AoV, aortic valve; PV, pulmonary vein.
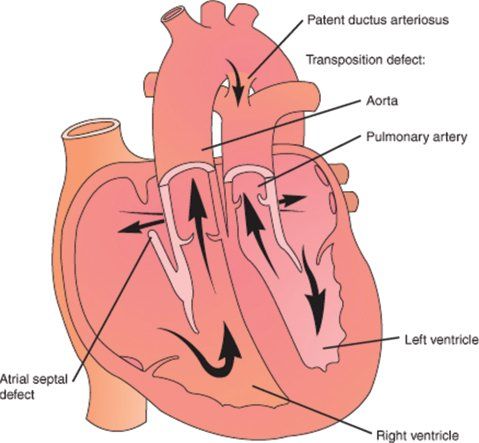
Figure 16.5. Schematic diagram of d-transposition of the great arteries (d-TGA). (From Lippincott’s Nursing Advisor 2012. See: www.LippincottSolutions.com )
COMPLICATIONS
The preferred operative approach in the current era for management of d-TGA is the arterial switch operation (ASO). Thus, any anatomical feature that makes performing an ASO difficult or impossible is important for the echocardiographer to identify. In this operation, both great vessels are transected, the branch pulmonary arteries are brought anterior to the neoaortic root (LeCompte maneuver), the coronary arteries are transferred from the native aortic root to the neoaortic root, and the great vessels are reanastomosed in the “switched” position (Fig. 16.6). Any significant atrial or ventricular septal defect is closed. Obstruction of either outflow tract, abnormalities of the pulmonary valve (which would function as the neoaortic valve after ASO), certain coronary patterns, nonfacing sinuses of the semilunar valves, and straddling of the tricuspid valve through a VSD (Video 16.4) are examples of such anatomical features that may complicate an ASO. Multiple muscular VSDs may be difficult to close and thus affect the management strategy. Aortic arch obstruction, while uncommon, needs to be identified so that it can be appropriately addressed at surgery.
Late clinical presentations of d-TGA raise additional concerns. First, if the left ventricular systolic pressure is low, concern is raised about the ability of the left ventricle to handle an acute transition to the pressure load of the systemic circulation. However, echocardiographic parameters of left ventricular shape or mass have not been shown to predict outcome after a late arterial switch, although extrapolated parameters have been used in surgical decision making. Second, pulmonary vascular disease is known to develop at an accelerated rate in patients with d-TGA and should be a consideration in patients over several months of age.
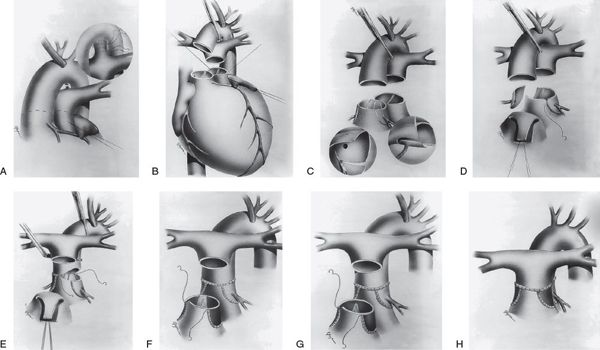
Figure 16.6. Surgical technique of the arterial switch operation. A: The ductus arteriosus is divided between suture ligatures and the branch pulmonary arteries are dissected out to the hilum to provide adequate mobility for anterior translocation. B: Transection of the great arteries. The left ventricular outflow tract, neoaortic valve, and coronary arteries are thoroughly inspected. C: The coronary arterial buttons are excised from the free edge of the aorta to the base of the sinus of Valsalva. D: The coronary buttons are anastomosed to V-shaped excisions made in the aorta. E: The pulmonary artery is brought anterior to the aorta (LeCompte maneuver). Anastomosis of the proximal neoaorta is shown. F: The coronary donor sites are filled with autologous pericardial patches. Two separate patches (F) or a single U-shaped patch (G) may be used. G: Anastomosis of the proximal neopulmonary artery and distal pulmonary artery. H: Completed anastomosis of the proximal neopulmonary artery and the distal pulmonary artery. (Reprinted from Wernovsky G. Transposition of the great arteries. In: Allen HD, Driscoll DJ, Shaddy RE, et al, eds. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents. 7th ed. Philadelphia: Lippincott Williams & Wilkins; 2008:1038–1087, with permission. Permission also obtained from the original source: Sabiston DC Jr, Spencer FC, eds. Surgery of the Chest. Philadelphia: WB Saunders; 1990:1435–1446.)
The echocardiographer has an important role in evaluating patients who have previously undergone surgery for d-TGA. The ASO became the predominant surgical strategy at most institutions by the late 1980s, and thus the longest published longitudinal follow-up data available are 15 to 20 years after ASO. As such data emerge, it has become apparent that important postoperative issues include coronary occlusion (symptomatic or asymptomatic) in 3% to 14% of patients, stenosis at the great vessel anastomoses (supravalvar pulmonic stenosis being more common than supravalvar aortic stenosis [5% to 30% versus 2% to 5%]), neoaortic root dilation in about 50%, neoaortic insufficiency (trivial to mild, about 30%; moderate to severe, 1% to 7%), and uncommonly, clinically significant bronchopulmonary collateral vessels. Rarely, patients who underwent ASO in the neonatal period present later with pulmonary arterial hypertension; the etiology of this phenomenon remains unclear.
Before the ASO era, patients were managed with an atrial level “switch,” using either the Senning or Mustard technique. These two techniques involve rerouting systemic venous drainage to the mitral valve and pulmonary venous drainage to the tricuspid valve by way of intra-atrial baffles. The circulation is physiologically “corrected,” but the right ventricle serves as the systemic ventricle while the left ventricle serves as the pulmonary ventricle. Late complications include sinus node dysfunction and other atrial arrhythmias (40% to 60%), baffle obstruction or leak (5% to 31%), right ventricular dysfunction (about 60% at 25-year follow-up), tricuspid regurgitation, and left ventricular outflow tract obstruction.
BASICS OF ECHOCARDIOGRAPHIC ANATOMY AND IMAGING
Classic Two-Dimensional Echocardiographic Anatomy and Hemodynamics
The diagnosis of d-TGA is established by demonstrating normal atrial situs, atrioventricular alignments, and ventricular looping, in association with ventrículoarterial discordance. The aorta arises from the right ventricle, while the pulmonary artery arises from the left ventricle. In most cases, the aortic valve is anterior and to the right of the pulmonary valve, and there is characteristically fibrous continuity between the pulmonary and mitral valve. With this fundamental anatomy established, the sonographer can then proceed to evaluate additional key anatomical features such as the presence or absence of VSD(s), coronary artery pattern, outflow tract and semilunar valve anatomy, and aortic arch anatomy (Video 16.2).
Beginning with a two-dimensional subcostal frontal sweep allows the sonographer to establish atriovisceral situs, atrioventricular alignments, and ventricular looping (Fig. 16.7). As the sweep is extended to the outflow tracts and great vessels, the more posterior semilunar valve arising from the left ventricle is seen to give rise to a great vessel that bifurcates, consistent with the pulmonary artery. The more anterior great vessel arises from the right ventricle and does not bifurcate, consistent with the aorta. This initial sweep also allows the sonographer to glean preliminary information about the size of the interatrial communication and the presence or absence of a VSD. Two-dimensional subcostal short-axis sweeps provide further opportunities to evaluate the atrial and ventricular septa and ventricular morphology and to visualize the parallel orientation of the great vessels (Fig. 16.8). The size of any interatrial communication or VSD should be measured. The coronary arteries may be visualized from various subcostal views. In particular, the subcostal frontal sweep is useful when the circumflex coronary artery arises from the right coronary artery and has a retropulmonary course, which is the second most common coronary artery arrangement in d-TGA (Fig. 16.9; Video 16.3). In this situation, the circumflex is typically well seen due to its perpendicular orientation to the plane of sound. Its position anterior to the mitral annulus but posterior to the pulmonary valve can be established. With proper transducer angulation, a substantial length of circumflex coronary artery can be viewed as a “train track.” Information about coronary arterial anatomy from subcostal views is usually complementary to that from the parasternal short-axis view. Color Doppler evaluation from subcostal views further defines shunting across an interatrial communication or VSD and provides information about atrioventricular and semilunar valve function (Fig. 16.10). Spectral Doppler interrogation of the PFO or ASD (with mean gradient) should be obtained from the subcostal frontal or short-axis view as well as spectral Doppler interrogation of the outflow tracts and semilunar valves.
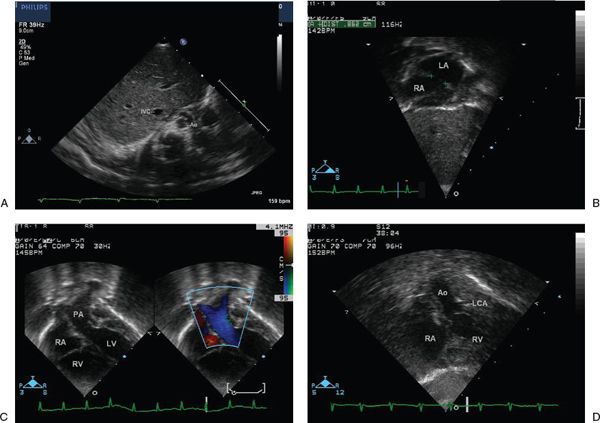
Figure 16.7. Subcostal four-chamber view. A: Transverse view through the liver demonstrates normal visceral situs with normal position of the inferior vena cava and aorta. IVC, inferior vena cava; Ao, aorta. B: View of the atria demonstrates the atrial situs and the atrial septal defect. In this case, a large communication after balloon atrial septostomy is demonstrated. LA, left atrium; RA, right atrium. C: With continued anterior angulation, the left ventricle is seen to give rise to the pulmonary artery, which bifurcates into the right and left branch pulmonary arteries. PA, pulmonary artery; RA, right atrium; RV, right ventricle; LV, left ventricle. D: With extreme anterior angulation, the right ventricle is seen to give rise to the aorta, which courses superiorly and does not bifurcate. Ao, aorta; LCA, left coronary artery; RA, right atrium; RV, right ventricle.
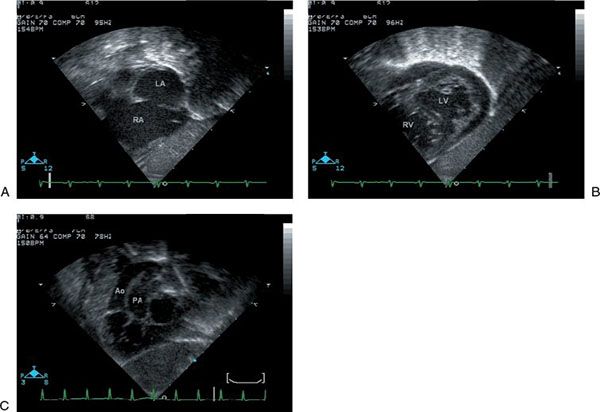
Figure 16.8. Subcostal short-axis view. A: The right and left atria and the atrial septal defect are well seen. LA, left atrium; RA, right atrium. B: Sweeping toward the apex, the ventricles are seen. The left ventricle has a characteristically smooth septal surface and two mitral valve papillary muscles are seen. A muscular ventricular septal defect is noted. LV, left ventricle; RV, right ventricle. C: The great vessels are seen in parallel with the aorta anterior and arising from the right ventricle. Ao, aorta; PA, pulmonary artery.
For sonographers who start the study from a parasternal long-axis view, the characteristic parallel orientation of the two great vessels is readily apparent (Fig. 16.11), although this view does not provide as ready assessment of the normalcy of other aspects of the anatomy as the subcostal approach does. Pulmonary–mitral valve fibrous continuity is usually present in this view. From the parasternal long-axis view, the aortic and pulmonary annuli should be measured in systole; the pulmonary valve annulus is normally slightly larger than the aortic valve annulus. Any abnormalities of the semilunar valve structure or function should be noted. Outflow tract obstruction or a malalignment-type VSD should be readily apparent. Color Doppler evaluation of all four cardiac valves should be performed.
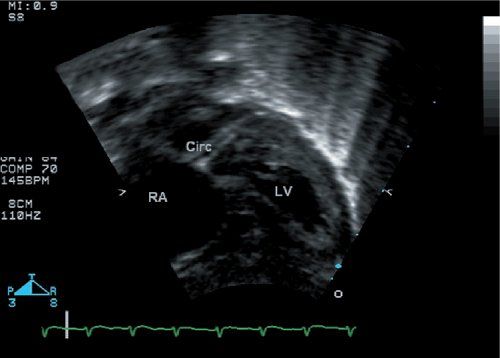
Figure 16.9. The circumflex coronary artery is well seen in this subcostal four-chamber view. Watching this entire imaging sweep allows one to identify that the circumflex courses posterior to the pulmonary artery. RA, right atrium; LV, left ventricle.
Turning to the parasternal short-axis view, one notes the relative position of the aortic and pulmonary valves (Fig. 16.12). Typically, the aortic valve is anterior and slightly rightward of the pulmonary valve annulus, although the positions can range along a continuum from the aortic valve directly anterior to the pulmonary valve, to side-by-side great vessels (aortic valve rightward) to even an anterior leftward aortic valve position in a minority of cases. Any structural abnormality of the aortic or pulmonary valve should be noted. The intercoronary commissure of the aortic valve is most commonly directly aligned with a commissure of the pulmonary valve (Fig 16.12A). If this is not the case (Fig 16.12B), this finding should be communicated to the surgeon as it may complicate the coronary transfer.
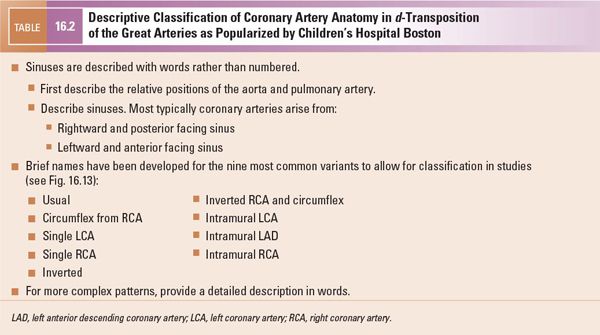
The parasternal short-axis view is also the primary view from which the coronary arterial pattern is determined. This information is of paramount importance for surgical planning as certain coronary artery patterns may add significantly to the technical difficulty of the operation (see also “Key Findings that Alter Management” later). Several nomenclature schemes have been developed to describe coronary artery patterns in d-TGA; the most commonly used nomenclatures are the Leiden convention (Table 16.1) and the descriptive approach popularized by Boston Children’s Hospital (Table 16.2, Fig. 16.13). Yacoub and Radley-Smith also developed a nomenclature scheme in the 1970s (Types A through F), but this scheme is not comprehensive and is not further described in this text. The most common coronary artery patterns are shown in Figures 16.13, 16.14, and 16.15 (Videos 16.2, 16.3, 16.5). Table 16.3 gives the relative frequencies of the most common coronary patterns. If coronary arterial anatomy is difficult to resolve, moving up one or two interspaces from the usual parasternal short-axis view (“high parasternal” view) may be helpful. The parasternal long-axis view, apical view, and subcostal views often add valuable complementary information (Figs. 16.9 and 16.16). Color Doppler with a low Nyquist value (usually 15–30 cm/s) should be used to confirm the appropriate direction of flow within structures thought to be the coronary arteries.
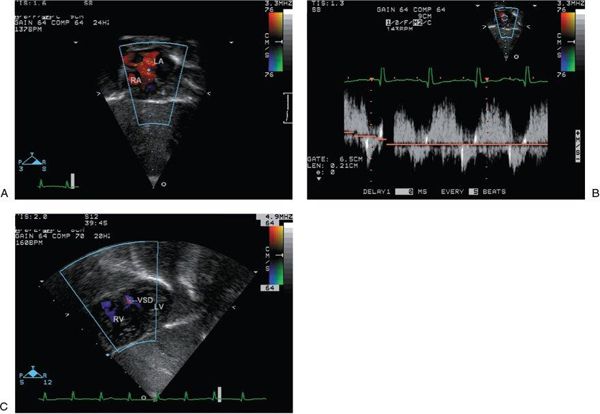
Figure 16.10. Subcostal views with the addition of color Doppler. A: Color flow Doppler across the atrial septal defect from the subcostal four-chamber view demonstrates the direction of shunting, which at this moment in time is left to right. LA, left atrium; RA, right atrium. B: Spectral Doppler interrogation of the atrial septal defect demonstrates low-velocity, bidirectional shunting. C: Color flow Doppler across the ventricular septum from the subcostal short-axis view demonstrates a small muscular ventricular septal defect. VSD, ventricular septal defect; RV, right ventricle; LV, left ventricle.
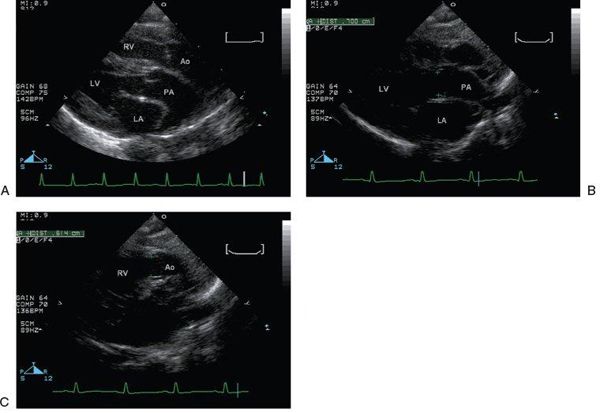
Figure 16.11. Parasternal long-axis view. A: The great vessels are seen in parallel. RV, right ventricle; Ao, aorta; LV, left ventricle; LA, left atrium; PA, pulmonary artery;. B: The pulmonary valve annulus measures 7 mm (green pluses). LV, left ventricle; LA, left atrium; PA, pulmonary artery. C: The aortic valve annulus (6 mm) (green pluses) is normally slightly smaller than the pulmonary valve annulus. RV, right ventricle; Ao, aorta.
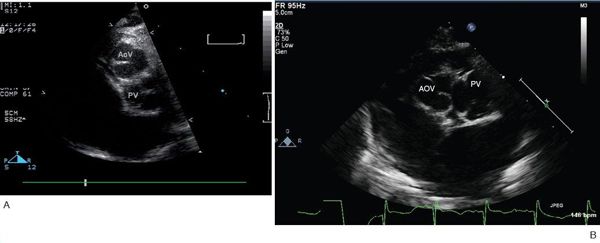
Figure 16.12. Parasternal short-axis view. A: Typical relative positions of the aortic and pulmonary valves, with the aortic valve anterior and slightly rightward. Note that the facing commissures of the two valves are aligned. AoV, aortic valve; PV, pulmonary vein. B: In this patient, the commissures of the semilunar valves are not aligned. AoV, aortic valve; PV, pulmonary vein.

A careful inspection of the ventricular septum for VSD(s) with two-dimensional imaging is also performed in the parasternal short-axis view (Fig. 16.17). If a VSD is present, its anatomical type is determined. Color Doppler interrogation of the ventricular septum confirms the presence or absence of VSD(s) as well as the direction of flow, which is frequently bidirectional in the neonate with d-TGA. Since right and left ventricular systolic pressures will be very similar in the neonate, using low Nyquist limits (60 cm/s or lower) will help detect low-velocity ventricular-level shunts. If a VSD is present, spectral Doppler interrogation of the VSD jet allows estimation of the transventricular pressure gradient.
The apical views are most useful in d-TGA for assessing valvar function and ensuring normal ventricular sizes (Fig. 16.18). The great vessel arising from the left ventricle can be visualized to bifurcate, demonstrating that it is the pulmonary artery. The structure and function of the atrioventricular and semilunar valves are assessed with two-dimensional, color Doppler, and spectral Doppler imaging. The apical view can also add some confirmatory information about coronary artery anatomy, particularly in the coronary variant in which the circumflex coronary artery arises from the right coronary artery and passes posterior to the pulmonary root. This course of the circumflex coronary artery can be seen on an apical sweep from posterior to anterior.
Long-axis suprasternal notch imaging provides the best view of the ductus arteriosus to assess its patency and size by two-dimensional imaging (Fig. 16.19). In addition, the aortic arch should be carefully inspected to rule out transverse arch hypoplasia or coarctation. Color flow Doppler demonstrates the direction of ductal flow as well as flow through the aortic arch. Pulsed-wave spectral Doppler interrogation within the ductus arteriosus further clarifies the pattern of shunting, which is typically predominantly from the aorta to the pulmonary artery. Short-axis suprasternal notch imaging may be useful in clarifying coronary artery anatomy as well.
Basic Imaging of d-TGA—Challenges
Generally, identification of d-TGA is straightforward for any sonographer who has at least some experience with congenital heart disease. Delineation of the coronary artery anatomy is much more challenging, given the small size and quite anterior location of the coronary arteries. Higher-frequency transducers with lower dynamic range settings may make the coronary arteries easier to visualize. At times, one sees linear, echo-free spaces in the vicinity of the semilunar valves, which are easily confused with the true coronary arteries. Confirmation that a structure truly represents a coronary artery can be made by demonstrating color Doppler flow with appropriate direction within the space and acquiring confirmatory images from other views. Coronary arteries that arise higher than usual (at the sinotubular junction or above) will be difficult to visualize in the expected parasternal short-axis view, but may be identified by sweeping more cranially in the parasternal short-axis view or from the parasternal long-axis view (Fig. 16.20).
Ventricular septal defects, particularly small muscular ventricular septal defects, can be easily overlooked when ventricular pressures are equal, particularly in the presence of large patent ductus arteriosus or additional large ventricular septal defect. Careful inspection of the 2D images of the ventricular septum and color flow assessment of the entire muscular septum are necessary (see Figs. 16.8B, 16.17).
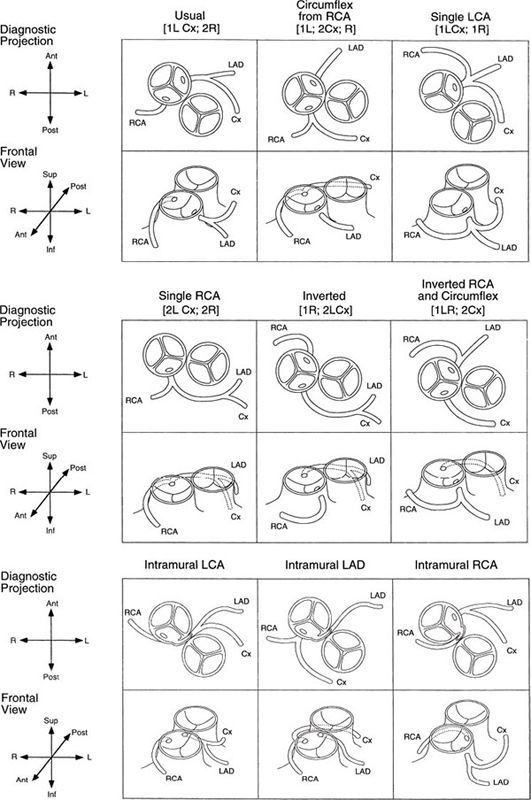
Figure 16.13. Coronary artery patterns in d-transposition of the great arteries (d-TGA). Nomenclature for each pattern (Children’s Hospital Boston and Leiden conventions) is given in each set of images. Top: Diagnostic projection; diagram of the origin and proximal course as visualized by two-dimensional echocardiography and caudally angulated aortography. Bottom: Same coronary artery distribution as viewed anteriorly (surgeon’s view, frontal projection). Note that the circumflex coronary artery, or even the entire left coronary artery system, is more likely to pursue a retropulmonary course when the great arteries are in a side-by-side relationship. (Reprinted from Wernovsky G. Transposition of the great arteries. In: Allen HD, Driscoll DJ, Shaddy RE, et al, eds. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2012:1097–1046, with permission. Permission also obtained from the original source: Wernovsky G, Sanders SP. Coronary artery anatomy and transposition of the great arteries. Coron Artery Dis. 1993;4:148–157.)
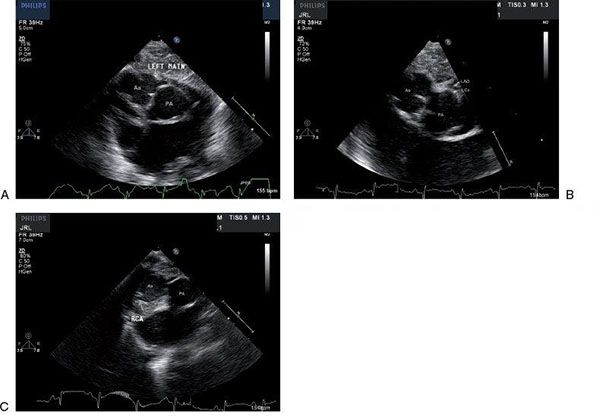
Figure 16.14. Two-dimensional parasternal short-axis views of the most common coronary artery pattern, termed “usual” or by the Leiden classification [1 AD, Cx; 2 R]. A: The origin of the left coronary artery is from the leftward sinus that is adjacent to or “facing” the pulmonary valve. Slight angulation away from a standard parasternal short-axis view may be necessary to demonstrate the origin of the vessel. Ao, aorta; PA, pulmonary artery. B: The bifurcation of the left coronary artery cannot be demonstrated in the same still frame as the origin but is well seen here. Ao, aorta; PA, pulmonary artery; left anterior descending coronary artery. C:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


