Course and Treatment of Chronic Obstructive Pulmonary Disease
In past decades, the treatment of chronic obstructive pulmonary disease (COPD) has been approached by many physicians and patients alike with a nihilistic attitude, assuming that the disease was progressive, incurable, and untreatable. More recently, as our understanding of the clinical epidemiology and value of therapy of COPD has improved, this attitude has changed. Physicians have come to approach COPD in the same way as other chronic diseases, such as diabetes, rheumatoid arthritis, and coronary artery disease. With modern comprehensive treatment, the diagnosis of COPD is compatible with prolonged survival, good quality of life, and independent functional status for many who have this illness. The purpose of this chapter is to summarize the current understanding of the course of COPD and best approaches to treatment.
OVERVIEW OF COPD
COPD is a disorder that is characterized by slow emptying of the lung during a forced expiration. In practice, this is measured as the forced expiratory volume in 1 second/forced vital capacity (FEV1/FVC) ratio, and the arbitrary definition of airflow obstruction is generally taken to be an FEV1/FVC ratio lower than 0.70.1 Because the rate of emptying of the lung falls with advancing age, many elderly individuals demonstrate airflow obstruction even in the absence of a clinical diagnosis of COPD. For this reason, an alternative criterion to define airflow obstruction incorporates lower limit of normal thresholds instead of the fixed ratio criteria.2 Several disorders cause chronic airflow obstruction—long-standing asthma, cystic fibrosis, bronchiectasis, bronchiolitis obliterans, lymphangioleiomyomatosis, panbronchiolitis, silicosis, Sjögren syndrome, and diffuse interstitial processes such as eosinophilic granuloma and sarcoidosis. The diagnosis of COPD is usually limited to individuals who have chronic airflow obstruction associated with tobacco smoke or some other noxious inhalant, and it is usually not difficult to distinguish it from other causes of chronic airflow obstruction. The most commonly associated clinical disorders associated with COPD are emphysema and chronic bronchitis. Emphysema is defined anatomically by airspace enlargement due to disappearance of alveolar septae (see Chapter 39). This leads to the characteristic loss of elastic recoil, which, in turn, causes slowing of airflow from the lungs, hyperinflation, and air trapping (see Chapter 40). Chronic bronchitis is characterized by chronic cough and sputum production, which is present in about one out of three people with early COPD. Chronic cough and sputum production in cigarette smokers is often, but not always, associated with chronic airflow obstruction. When chronic mucus hypersecretion is associated with airflow obstruction, it is often called chronic obstructive bronchitis. The anatomic correlates of chronic bronchitis are mucus gland hyperplasia and goblet cell metaplasia in large- and medium-sized airways.3 Patients with COPD also have small- and medium-sized airway involvement with inflammation, narrowing, tortuosity, mucus plugging, and fibrosis that contributes to the airflow limitation. As the disease evolves, there is obliteration of small airways. Some patients with a long-standing history of asthma develop airflow obstruction that is not completely reversible, episodes of cough and wheeze, and chronic sputum production. These individuals are often classified as having chronic asthmatic bronchitis and tend to have a somewhat better prognosis for survival than those with typical tobacco-related COPD. Physicians have a tendency to classify women as having asthma and men as having COPD despite similar medical histories.
NATURAL HISTORY OF COPD
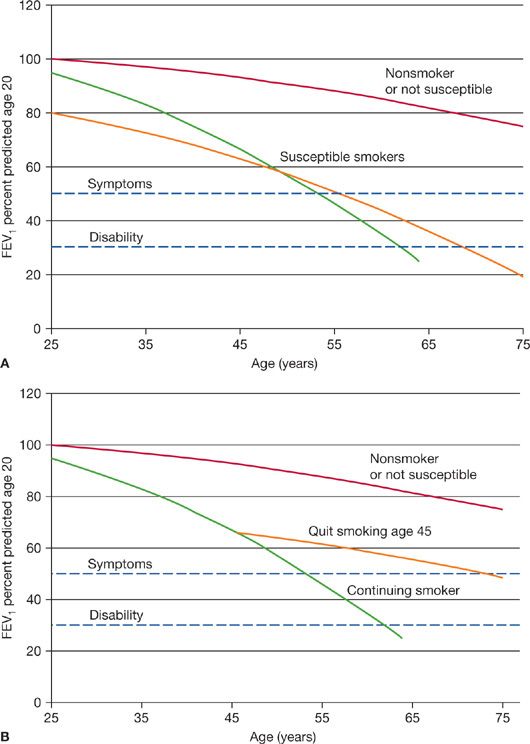
COPD results from an increase in the rate of decline in lung function over time. Normal nonsmoking adults lose FEV1 at a rate of 30 mL/yr, thought to be the consequence of the aging-related loss of elastic recoil of the lung. Persons who develop COPD may start in early adulthood with lower levels of lung function and also have increased rates of decline.4,5 Studies of patients with COPD show an average annual decline in FEV1 of 45 to 69 mL/yr (Fig. 42-1A). However, there may be considerable heterogeneity between patients and over time.6,7 This leads to the insidious loss of ventilatory reserve capacity that often is asymptomatic and unrecognized by patients and physicians alike. Chronic bronchitis may be dismissed as an innocent “smoker’s cough” because patients fail to understand that it is abnormal to produce daily sputum. As ventilatory reserve decreases, people with mild COPD tend to limit strenuous activities, so breathlessness with activities of daily living is not ordinarily an early symptom of the disease. When the ventilatory reserve decreases to the extent that mild exertion such as climbing stairs, bed making, or carrying groceries is limited, patients tend to seek medical advice. In some cases, the first clinical presentation of disease is an acute episode of bronchospasm, dyspnea, or even respiratory failure in association with a respiratory infection or exposure to respiratory irritants. Thus, the onset of COPD may appear precipitous even though it is the cumulative result of decades of progression.
Figure 42-1 A. The natural history of COPD is presented for three hypothetical individuals. Pulmonary function is plotted as the percent of predicted lung function for a young adult who has attained maximal lung growth. Those who do not smoke, or are not susceptible to cigarette smoke typically lose about 25% of their young adult lung function throughout life. Individuals are susceptible to the adverse effects of smoking because of increased decline of lung function, or low lung function in young adult life. Although the abnormality of lung function is detectable for many years, symptoms do not develop until there is loss of approximately 50% of lung function (upper dashed line), which occurs in middle age or later. If the disease progresses, it may lead to substantial disability within a decade of the onset of symptoms (lower dashed line). B. The natural history of COPD is displayed for a hypothetical continuing smoker, and an individual who quit smoking at age 45. The axes are identical to those in (A). If an individual ceases smoking in the asymptomatic phase of COPD, the rate of decline of lung function reverts toward normal. In this example, the detection of abnormal lung function and cessation of smoking has a substantial effect of delaying the onset of respiratory symptoms. This plot is modified from the work of Fletcher and Peto and is commonly referred to as Fletcher curves. (Reproduced with permission from Fletcher C, Peto R. The natural history of chronic airflow obstruction. Br Med J. 1977;1(6077):1645–1648.)
People who discontinue smoking with mild to moderate degrees of airflow obstruction cease the rapid decline in FEV1, and have better survival (Fig. 42-1B).8 The improvement in survival depends largely upon the stage of disease. Persons who quit smoking with earlier disease have better outcomes compared with those who continue to smoke or those who quit smoking later in the disease. Once the disease is advanced, the inflammatory response persists and the proportional loss of lung function tends to progress. Because there are many years of asymptomatic decline in lung function, it is possible to diagnose COPD with forced expiratory spirometry before the disease is apparent and implement aggressive smoking intervention programs. There is a consensus that smokers with respiratory symptoms should be tested for COPD with spirometry. However, there is debate whether it is of value to screen for COPD among all cigarette smokers.9 Opponents of using spirometry for case-finding argue that the finding of a normal test would not alter physician behavior because all smokers should be encouraged to quit. It has also been argued that a normal spirometry test might provide a false sense of complacency for active smokers. Those who support the use of spirometry for COPD case-finding argue that early detection and aggressive smoking intervention have been proved to halt disease progression and improve survival, and the finding of abnormal spirometry may encourage patients and healthcare professionals to be more aggressive with smoking cessation. Moreover, some evidence points to the benefits of drug therapy in terms of lung function decline and survival in patients with mild to moderate airflow obstruction.10–12 While these data arise from post hoc analysis, the findings are promising.
With the progression of COPD comes progressive exercise limitation.13 This is due to the increased work of breathing as ventilation increases with exercise. With increased respiratory rate, patients develop dynamic hyperinflation—a condition in which the end-expiratory lung volume does not return to the static end-expiratory volume of functional residual capacity (FRC). The hyperinflation that occurs causes increased work of breathing and exacerbates dyspnea. An indicator of dynamic hyperinflation is the inspiratory capacity (IC), which progressively falls with increasing ventilation. Measures that reduce dynamic hyperinflation, increasing IC, can improve exercise capacity. These include alterations in breathing pattern, oxygen supplementation, and use of inhaled bronchodilators.
As COPD progresses, ventilation–perfusion inhomogeneity causes an increase in the alveolar–arterial oxygen difference. Eventually, alveolar hypoxemia leads to pulmonary hypertension, which becomes manifested as cor pulmonale. The alveolar hypoxemia may be compounded by alveolar hypoventilation—manifested by arterial hypercapnia. Physical findings indicative of cor pulmonale are venous engorgement, edema, and physical findings of pulmonary hypertension and right ventricular failure including accentuated pulmonic second heart sound, right ventricular heave, tricuspid regurgitation murmur, hepatojugular reflux, and ascites. Chest imaging shows central enlargement of the pulmonary arteries. Once cor pulmonale is clinically apparent, survival is markedly reduced in proportion to the elevation of pulmonary artery pressure. Chronic respiratory failure is defined by chronic hypoxemia (sea-level resting PaO2 ≤60 mm Hg or 8 kPa) with or without attendant hypercapnia (PaCO2 >45 mm Hg).
Patients with advanced COPD may restrict their activities to a bed-and-chair lifestyle because of severe exercise incapacitation. This limitation can lead to social isolation, depression, and skeletal muscle deconditioning, which, in turn, further restrict activity and impair quality of life. Protein and calorie malnutrition occurs as the consequence of impaired nutritional intake caused by dyspnea.14 Malnutrition is augmented by increased metabolic demands caused by increased basal oxygen consumption, inefficient skeletal muscle oxygen utilization, and cachexia-producing cytokines such as tumor necrosis factor-α (TNF-α).15
DIAGNOSIS OF COPD
Physical examination and chest imaging are insensitive methods for diagnosis of COPD. Physical findings of hyperinflated lungs such as low-lying diaphragm, decreased breath sounds, and hyperresonant chest percussion are highly specific for COPD, but usually only in advanced disease.16 One study has suggested that a distance between the thyroid cartilage and the sternal notch less than 4 cm in a smoker older than age 45 is highly indicative of the presence of COPD.17 Clubbing of the fingers is rare in COPD and, if present, suggests another diagnosis such as bronchiectasis, asbestosis, or lung cancer. High-resolution computed tomography (HRCT) of the lung is useful in establishing the presence of emphysema. Quantitative analysis of HRCT is a promising technique for early detection of emphysema. α1-Antitrypsin deficiency is an uncommon, but not rare, condition associated with premature emphysema. Testing for α1-antitrypsin deficiency is indicated in those most likely to have the disorder (see Chapter 40 and Table 42-1). Some experts advise that all patients with COPD should be tested for α1-antitrypsin deficiency because treatments are available for those with the most severe form of deficiency.18 HIV/AIDS is also associated with premature emphysema and accelerated lung function decline,19,20 and screening for HIV should be performed for persons with emphysema and HIV risk factors such as intravenous drug use or high-risk sexual activity.
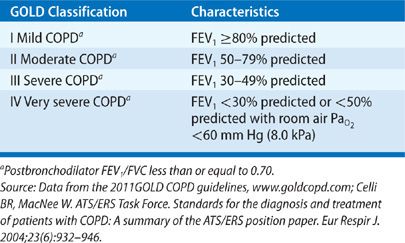
The diagnosis of COPD, classification of its severity, and progression of the disease can be monitored with spirometry, a simple, noninvasive, and inexpensive test. The FEV1/FVC ratio, reflecting the rate of emptying of the lung, is used to define the presence of an obstructive ventilatory defect, commonly defined as a ratio less than 0.70 or below the lower limit of normal. Once airflow obstruction is established, the severity of the airflow limitation is classified by the reduction of FEV1 compared with a healthy reference population. Table 42-2 shows the widely used GOLD classification of severity based on the FEV1. Lung volume measurements, by plethysmography, helium dilution, nitrogen washout, or single-breath methods typically show hyperinflation (elevated TLC) and air trapping (elevated residual volume [RV]), and thus are useful to exclude restrictive lung diseases. The carbon monoxide diffusing capacity (DCO) is an indicator of emphysema and is roughly inversely correlated with the anatomic extent of emphysema in patients who have an FEV1 greater than 1.0 L.
CLASSIFICATION OF COPD
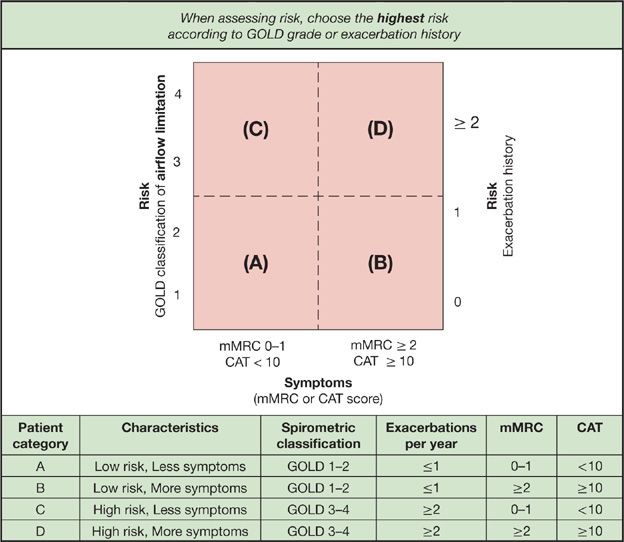
Classification of COPD involves determination of symptoms and assessment of risk. Symptoms are determined using standardized questionnaires (see Table 42-3 and www.catsonline.org), while risk assessment is defined by severity of ventilatory limitation and exacerbation history. Based on symptoms and risk of exacerbations, individuals are grouped into one of four different patient categories (see Fig. 42-2). The categories are informative for determining prognosis and treatment of COPD. Additional instruments are available for quantifying the impact of COPD.21
Figure 42-2 Combined COPD assessment. An understanding of the impact of COPD on an individual patient combines assessment of symptoms and future risk of exacerbation. To use this figure, first assess symptoms with the modified medical research council (mMRC) or COPD assessment test (CAT) scale and determine if the patient has less symptoms (mMRC <2 or CAT <10) or more symptoms (mMRC ≥2 or CAT ≥10). Next, assess the risk of future exacerbation by determining prior exacerbation history and severity of airflow limitation with high risk of future exacerbation in individuals with GOLD airflow classification 3 to 4 or ≥2 exacerbations in the prior year (future risk should be determined by the method indicating higher risk). With this figure, individuals are stratified into one of four categories (A, B, C, D). which helps describe the burden of disease and informs potential treatments. (Reproduced with permission from Global Strategy for the Diagnosis, Management, Prevention of COPD, © Global Initiative for Chronic Obstructive Lung Disease (GOLD), all rights reserved. Available from http://www.goldcopd.org.)
PROGNOSIS OF COPD
The prognosis in COPD may vary widely. Physicians are poor prognosticators of survival in COPD.22 In part, this is because the disease is one of widely varying rates of progression, and, in part because death is often due to susceptibility to intercurrent illness and other smoking-related illness such as lung cancer rather than progressive respiratory failure. Recent studies have demonstrated heterogeneity in lung function decline, with some patients with COPD having little or no decline in FEV1 over time.6,7
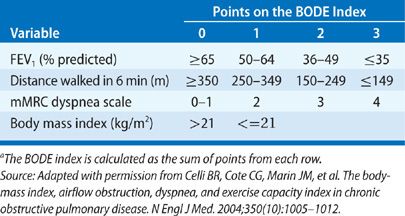
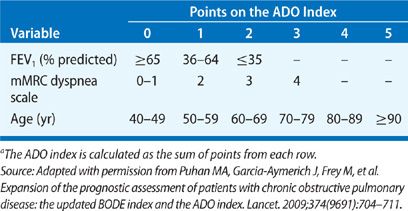
Several factors have been identified that predict poor survival in COPD. These include low FEV1, active smoking status, hypoxemia, poor nutrition, the presence of cor pulmonale, resting tachycardia, low exercise capacity, severe dyspnea, poor health–related quality of life, anemia, frequent exacerbations, comorbid illnesses, and low DCO.23 Patients with an FEV1 less than 35% predicted have about 10% mortality per year.24 If a patient reports that they are unable to walk 100 m without stopping because of breathlessness, the 5-year survival is only 30%.25 A multidimensional prognostic index that takes into account several indicators of COPD prognosis is the BODE index (body mass index [BMI], obstructive ventilatory defect severity, dyspnea severity, and exercise capacity).26 See Table 42-4 for calculation of the BODE prognostic score. The components are derived from measures of the BMI (weight in kg/height m2), FEV1 percent predicted, and the modified Medical Research Council dyspnea score (Table 42-3). A BODE score greater than 7 is associated with a 30% 2-year mortality; whereas a score of 5 to 6 is associated with 15% 2-year mortality. If the BODE score is less than 5, the 2-year mortality is less than 10%. In settings where the 6-minute walk test is not available, the ADO index (age, dyspnea and obstruction) also provides useful prognostic information (Table 42-5).27 The ADO index ranges from 0 to 10 points, with each point increase in the index associated with a 42% increase in odds of death at 3 years for patients with longstanding and severe COPD.
TREATMENT OF STABLE COPD
The goals of treatment of COPD are to prevent progression and complications of the disease, relieve symptoms, improve exercise capacity, improve quality of life, treat exacerbations, and improve survival.28,29 Efforts are being made to standardize the most optimal treatment guidelines for COPD.30
 EDUCATION
EDUCATION
The diagnosis of COPD can be a life-changing event for people, so understanding the nature and prognosis of the disease is an important and underemphasized aspect of care. There is a wide divergence of understanding of the implications of having COPD, and many patients do not understand that COPD comprises both the diagnoses of emphysema and chronic bronchitis. Table 42-6 lists topics that should be discussed with COPD patients. It is neither possible nor effective to cover all of these topics in a single session, so several sessions with repetition and expansion of the educational messages are necessary. Supplemental written materials or referral to a health educator is also necessary for many patients. Local and national volunteer health organizations provide useful educational materials and group educational sessions (e.g., www.copdfoundation.org, www.lung.org). Special counseling is needed for patients with α1-antitrypsin deficiency and their family members to determine whether genetic testing is necessary or desired. In patients with advanced disease, discussions about end-of-life planning and advance directives regarding life support are often welcomed by patients and initiate discussions between the patient and family. Patients should be encouraged to discuss information that they obtain from newspapers or the Internet, as some may be instructive, but others are incorrect. Physicians should be prepared to deal with patients’ sense of guilt, as many view COPD as a self-induced disease. Caregivers need to address the reality that COPD is often stigmatized by patients, their families, and other healthcare providers. The physician should let the patient understand that nicotine dependence is a strong physical addiction and difficulty quitting smoking is not a measure of moral weakness or lack of will. The general message provided should be realistic, but positive. Current treatments for COPD can usually improve quality of life, restore activity levels, maintain social interactions, and reduce the frequency of complications.
 PREVENTION OF COPD PROGRESSION AND COMPLICATIONS
PREVENTION OF COPD PROGRESSION AND COMPLICATIONS
Presently, there are no proven treatments that prevent the progression of COPD in patients who continue to smoke cigarettes. Smoking cessation, however, does prevent the excessive decline in lung function and should be a primary goal for physicians caring for COPD patients. Patients with mild or moderate COPD may not know that they have underlying lung disease that can be halted by smoking cessation,4 or may adopt a fatalistic attitude that it is too late for help. Even severely impaired patients who are dyspneic at rest or use continuous oxygen may continue to smoke cigarettes or relapse after quitting. A smoking history should be obtained at each patient encounter because many patients fail to volunteer the extent of their smoking or report a smoking relapse following cessation. In patients who do smoke, achieving cessation should be a primary and persistent goal of treatment.31 Approaches to smoking cessation are given in detail in Chapter 41. For patients who do smoke, a direct, unambiguous, and personalized smoking cessation message should be given by the physician. The message should emphasize the harm of continued smoking, the benefits of cessation in terms of activities that are meaningful for the individual, and the understanding that smoking cessation is a realistic and achievable goal. Techniques of motivational interviewing are readily learned and are effective in changing health behaviors.32 Assistance with pharmacologic adjuncts such as nicotine replacement therapy, varenicline, or bupropion and referral to smoking cessation groups should be offered. Follow-up of smoking status and repeated smoking cessation messages should be performed at each encounter.
Exposure to respiratory irritants should be avoided in the workplace as well as the home. Although heavy occupational dust exposure rarely is the primary cause of COPD, exposure to dusty occupational jobs in smokers can increase the lung function deterioration from smoking and increase symptoms of cough and sputum.33 In developing countries, heavy exposure to particulates from burning of biomass fuels is associated with COPD, even in the absence of cigarette smoking.34 Efforts to improve indoor air quality may be effective in reducing symptoms and disease progression. Respiratory protective equipment should be worn by COPD patients exposed to heavy dust concentrations. There is no level of FEV1 that absolutely prohibits the use of respiratory protective equipment, but patients with COPD often experience untoward breathlessness with these masks because of the increased dead space and increased inspiratory resistance. Thus, many COPD patients need to change their work environment if they cannot tolerate protective devices. If COPD is complicated by allergy or overlaps with allergic asthma, environmental control measures should be instituted to the extent that these strategies are helpful. Smoking of marijuana and cocaine may cause airway irritation, and although there is no convincing evidence that they contribute to progression of COPD, their use ought to be discouraged.35
Pneumococcal vaccination is recommended, although the evidence of its particular efficacy in COPD is lacking.36 Annual influenza immunization can prevent or attenuate this potentially fatal infection. The killed vaccine is preferred, as cold-attenuated live influenza vaccines have not been approved for use in older patients and those with underlying lung disease. High-potency influenza immunization is recommended for older patients who may have an impaired immune response to the vaccine. During influenza epidemics, the use of neuraminidase inhibitors such as zanamivir and oseltamivir can minimize severity of infection if taken within 48 hours of onset of illness and are useful against both influenza A and B, and may limit the spread of infection. Peramivir, an injectable form of neuraminidase inhibitor, is now available for treatment of individuals with respiratory failure.
Replacement therapy with α1-antitrypsin should be considered for those individuals with severe deficiency. Observational studies suggest that individuals with moderate degrees of impairment (FEV1 35%–65% predicted) appear to benefit most in terms of preservation of lung function and improved survival.37 The human plasma–derived preparation of α1-antitrypsin is administered intravenously in a dose of 60 mg/kg weekly. Although the replacement treatment is derived from pooled human plasma, the risk of viral transmission is low and immunization for hepatitis B is not mandatory before initiating therapy.
 DRUG THERAPY
DRUG THERAPY
Over the past several decades, the evidence base for use of drug therapy in COPD has expanded, and provides an objective and generally optimistic picture that such treatment is effective. Bronchodilators and anti-inflammatory agents are used to reverse bronchoconstriction, improve lung function, improve quality of life, exercise capacity, and prevent exacerbations. Recent evidence however suggests that a combination of inhaled steroids and long-acting bronchodilators may improve survival as well as reduce exacerbations.38–40 Proposed future drug treatments that might alter progression of COPD are under active investigation, including inhibitors of cytokines, proteases, and oxidative stress. There is a poor correlation between the effect of bronchodilating drugs on lung function and symptom relief, so monitoring of treatment requires attention to patient-centered outcomes as well as lung function. Small amounts of bronchodilation can cause considerable improvement in functional capacity through decrease in dynamic exercise hyperinflation; and reduction in days of exacerbation can make considerable improvement in patients’ quality of life.
The usual approach to drug treatment for COPD is to sequentially add agents using the minimum number of agents and the most convenient dosing schedule, starting with the agents having the greatest benefit, best tolerance, and lowest cost. One approach to step-up therapy is provided in Figure 42-3. Inhaled bronchodilators are the foundation of treatment for COPD. They are given on a regular basis to maintain bronchodilation and on an as needed basis for relief of symptoms.41,42 Most breathless patients benefit from regular use of a maintenance bronchodilator. Both β-agonist and anticholinergic classes are available in short-duration (4–6 hours) and long-duration (12–24 hours) forms (Table 42-7). The choice of bronchodilator class and duration of effect depends upon the preference of the patient and the cost of the preparation. Combination of different classes of bronchodilators is often more effective than increasing the dose of a single agent, and combination inhalers can simplify treatment regimens. Patients with advanced COPD often use a combination of bronchodilators, including long-acting maintenance anticholinergics and β-agonists as well as symptomatic use of shorter-acting bronchodilators. Individuals with exacerbations may benefit from a combination inhaler of corticosteroids and long-acting bronchodilator. Long-acting oral preparations of theophylline are useful adjuncts in cases in which inhaled medication is too expensive or not acceptable for the patient. Chronic use of systemic corticosteroids should be reserved for individuals with very frequent or life-threatening exacerbations in those cases where discontinuation of steroids cannot be tolerated. Response to treatment is judged by symptomatic improvement, functional status, frequency of exacerbations, and spirometry. If patients are doing well for 6 to 12 months on a stable treatment regimen, then it is reasonable to see if a trial withdrawal of one of the drug components can be tolerated.