Emerging evidence suggests that neointimal degenerative changes with development of neoatherosclerosis (NA) may represent an important mechanism for late stent failure. The aim of the present study was to investigate the relation between degree of neointimal hyperplasia and incidence and characteristics of NA using optical coherence tomography. We identified a total of 252 stents with mean neointimal thickness (NIT) >100 μm in 212 patients: 100 bare metal stents (BMSs) and 152 drug-eluting stents (DESs). Based on the values of mean NIT, we divided stents into tertiles and compared neointimal characteristics among the 3 groups. NA was defined as the presence of lipid-laden intima and/or calcification inside the stent. In both BMS and DES, there was a difference in the prevalence of lipid-laden intima among the tertiles (18.2% vs 36.4% vs 47.1%, p = 0.042 [BMS]; 19.6% vs 56.9% vs 88.0%, p <0.001 [DES]). However, no difference in the prevalence of in-stent calcification was observed (21.2% vs 21.2% vs 2.9%, p = 0.053 [BMS]; 5.9% vs 9.8% vs 2.0%, p = 0.252 [DES]). In a multivariate model adjusting for stent type, follow-up duration, conventional coronary risk factors, statin, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blockade use, mean NIT was independently associated with the presence of NA (odds ratio 2.53, 95% confidence interval 1.96 to 3.27, p <0.001). This study demonstrates the presence of a positive correlation between degree of neointimal hyperplasia after stent implantation and presence of lipid-laden intima. This association is independent from stent type and time from implantation and suggests a possible pathogenic link between the two processes.
Emerging evidence suggests that neoatherosclerosis (NA) may play a role in the pathogenesis of late stent thrombosis, an infrequent but potentially catastrophic stent complication. Optical coherence tomography (OCT) is a near-infrared light-based imaging technology with high resolution, which allows accurate characterization of atherosclerosis in vivo, and has been extensively used to evaluate vascular response after stent implantation and atherosclerotic changes of neointima. In the present study, we used intracoronary OCT to evaluate the relation between the degree of neointimal hyperplasia (NIH) and the incidence and characteristics of NA.
Methods
From the Massachusetts General Hospital OCT Registry, 689 patients with previously implanted stents were identified from August 2010 to September 2012. Patients with incomplete demographic data (n = 18), unknown type of stent (n = 173), endothelial progenitor cell–capturing stents (n = 87), and polytetrafluoroethylene-covered stents (n = 1) were excluded from the study. Cases with any interventional procedure before imaging (n = 61), overlapping stents (n = 38), and stents with poor imaging quality (n = 40) were also excluded. Of the remaining 437 stents in 271 patients, we selected stents with a mean neointimal thickness (NIT) >100 μm for ≥3 consecutive 1-mm cross-sections to obtain a sufficient amount of neointima to detect tissue characteristics, as previously reported. Therefore, 252 stents in 212 patients were included in the final analysis: 100 bare metal stents (BMSs) and 152 drug-eluting stents (DESs). Hypertension was diagnosed when ≥1 of the following criteria was met: history of hypertension, use of antihypertensive drugs, systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg. Hyperlipidemia was diagnosed in case of history of hyperlipidemia, use of lipid-lowering drugs, or newly diagnosed hyperlipidemia according to institutional guidelines. Clinical and angiographic characteristics were compared between patients with and without NA. Based on the values of mean NIT, stents were divided into tertiles, and incidence and characteristics of NA were compared among the 3 groups. According to the follow-up duration, stents were assigned to an early (<9 months), intermediate (9 to 48 months), or late (>48 months) phase. The registry was approved by each institutional review board, and every patient provided informed consent.
OCT image acquisition was performed using commercially available frequency domain (C7-XR OCT Intravascular Imaging System; St. Jude Medical Inc., St. Paul, Minnesota) or time domain (M2/M3 Cardiology Imaging Systems; LightLab Imaging Inc., Westford, Massachusetts) OCT systems. The intracoronary OCT imaging technique has been previously described. All the images were de-identified, digitally stored, and submitted to the Massachusetts General Hospital OCT Registry Laboratory for analysis.
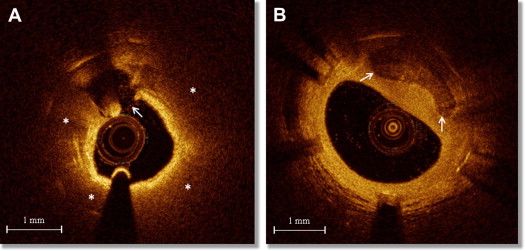
Cross-sectional OCT images were analyzed every 1 mm by 2 independent investigators (RV and TY) who were blinded to clinical and laboratory data. When there was discordance between the 2 observers, a consensus reading was obtained from a third investigator (KK). Quantitative and qualitative analyses were performed using OCT proprietary software for off-line analysis (LightLab Imaging Inc., Westford, Massachusetts). For each frame, lumen and stent areas were traced and minimum, maximum, and mean NIT were automatically calculated. NA was defined as the presence of lipid-laden intima and/or calcification inside the stent ( Figure 1 ). We defined lipid-laden intima as a diffusely bordered signal-poor region inside the stent ( Figure 1 ) and categorized it as lipid-rich intima (LRI) when lipid arc was wider than 90°. The percentage of LRI was calculated as (number of frames with LRI/total number of analyzed frames) × 100. When an LRI was covered by a fibrous cap thinner than 65 μm, the lesion was labeled as thin-cap fibroatheroma (TCFA)-like intima. In-stent calcifications were recorded as signal-poor or heterogenous regions with a sharply delineated border ( Figure 1 ). Neovascularization (NV) was defined as the presence of signal-poor holes or tubular structures with a diameter of 50 to 300 μm and categorized as peristrut and intra-intima, as previously reported. The presence of disrupted intima, defined as discontinuity of the fibrous cap connecting the lumen with the underlying lipid pool ( Figure 1 ), and thrombus, defined as a mass attached to the luminal surface or floating within the lumen, was also recorded.
Off-line quantitative analysis of coronary angiograms recorded at the time of OCT imaging was performed using a validated edge-detection system (CAAS Version 5.1; Pie Medical Imaging, Maastricht, The Netherlands). End-diastolic frames were selected for the analysis, and the tip of the catheter was used for calibration. Reference lumen diameter, minimum lumen diameter, diameter stenosis, and lesion length were measured.
Categorical variables were expressed as counts and percentage and compared using the chi-square or Fisher’s exact test, as appropriate. Continuous variables were expressed as mean ± SD and compared using the independent samples Student t test for 2-group comparisons and 1-way analysis of variance followed by Bonferroni post hoc test for 3-group comparisons. For the comparisons of OCT findings expressed as categorical values, the observed significance was further adjusted for the multiplicity of simultaneously conducted tests by Bonferroni correction. Mean NIT was evaluated as a continuous variable, in tertiles and quartiles. All the approaches showed similar results, and we chose for simplicity to present the results by tertiles. Intra- and interobserver variability was estimated by the k measure of agreement. Multivariate logistic regression analysis was performed to determine the impact of mean NIT on the presence of NA (dependent variable), after adjusting for stent type, follow-up duration, conventional coronary risk factors, statin, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blockade use. Univariate analysis was first performed, and all the variables exhibiting a p value <0.05 were entered en bloc in the multivariate model. A generalized estimating equations approach with exchangeable correlation structure was used to take into account the within-subject correlation because of the analysis of multiple stents in a single patient. A p value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS 17.0 (SPSS Inc., Chicago, Illinois).
Results
Baseline patient characteristics are listed in Table 1 . Each value was compared between patients with NA in ≥1 stent and patients without NA. Serum creatinine levels were significantly higher in patients with NA, as well as total cholesterol and low-density lipoprotein cholesterol levels. Compared with patients with NA, those without NA were more frequently on statin and angiotensin-converting enzyme inhibitor or angiotensin II receptor blockade therapy. There was no significant difference in clinical presentation between patients with and without NA. However, a diagnosis of acute coronary syndrome was more frequent in patients with DES than in those with BMS (23% vs 12%, p = 0.044). In patients presenting with acute coronary syndrome, the stented segment was the culprit lesion in 47% of cases.
| Variable | Overall (n = 212) | NA (n = 112) | Non-NA (n = 100) | p |
|---|---|---|---|---|
| Age (yrs) | 62.4 ± 10.8 | 63.5 ± 11.0 | 61.1 ± 10.4 | 0.093 |
| Men | 167 (79) | 86 (77) | 81 (81) | 0.454 |
| Hypertension | 149 (70) | 76 (68) | 73 (73) | 0.413 |
| Hyperlipidemia | 158 (74) | 82 (73) | 76 (76) | 0.642 |
| Diabetes mellitus | 85 (40) | 45 (40) | 40 (40) | 0.979 |
| Smoker | 44 (21) | 28 (25) | 16 (16) | 0.107 |
| Creatinine (mg/dl) | 1.06 ± 1.16 | 1.22 ± 1.57 | 0.89 ± 0.27 | 0.043 |
| Total cholesterol (mg/dl) | 157.0 ± 37.8 | 162.4 ± 36.8 | 151.4 ± 38.3 | 0.042 |
| LDL cholesterol (mg/dl) | 86.8 ± 29.3 | 91.9 ± 29.5 | 81.4 ± 28.4 | 0.013 |
| Triglycerides (mg/dl) | 161.7 ± 176.5 | 173.1 ± 199.6 | 150.1 ± 149.6 | 0.372 |
| Clinical presentation | 0.492 | |||
| ST elevation myocardial infarction | 4 (2) | 3 (3) | 1 (1) | |
| Non–ST elevation myocardial infarction or unstable angina pectoris | 36 (17) | 21 (19) | 15 (15) | |
| Stable angina pectoris | 172 (81) | 88 (79) | 84 (84) | |
| Statins | 180 (85) | 89 (79) | 91 (91) | 0.019 |
| Angiotensin-converting enzyme inhibitor or angiotensin II receptor blockers | 133 (63) | 63 (56) | 70 (70) | 0.039 |
Angiographic findings are listed in Table 2 . There was no difference in stent location between the NA and the non-NA groups. Compared with stents without NA, those with NA had significantly smaller minimum lumen diameter and greater diameter stenosis. Lesion length and reference lumen diameter were not different between the two groups.
| Variable | Overall (n = 252) | NA (n = 121) | Non-NA (n = 131) | p |
|---|---|---|---|---|
| Coronary artery with stent | 0.988 | |||
| Right | 91 (36) | 44 (36) | 47 (36) | |
| Left anterior descending | 116 (46) | 55 (45) | 61 (47) | |
| Circumflex | 45 (18) | 22 (18) | 23 (17) | |
| QCA analysis | ||||
| Lesion length (mm) | 10.8 ± 5.0 | 11.1 ± 5.6 | 10.4 ± 4.5 | 0.277 |
| Minimum lumen diameter (mm) | 1.63 ± 0.74 | 1.53 ± 0.75 | 1.72 ± 0.73 | 0.039 |
| Reference lumen diameter (mm) | 3.03 ± 0.65 | 3.00 ± 0.63 | 3.05 ± 0.66 | 0.518 |
| Diameter stenosis (%) | 47.0 ± 18.8 | 50.1 ± 19.2 | 44.2 ± 18.2 | 0.011 |
| OCT analysis | ||||
| Mean neointimal thickness (μm) | 370 ± 204 | 435 ± 203 | 313 ± 189 | <0.001 |
| Maximum neointimal thickness (μm) | 633 ± 327 | 766 ± 348 | 517 ± 257 | <0.001 |
| Minimum neointimal thickness (μm) | 166 ± 148 | 184 ± 146 | 149 ± 149 | 0.069 |
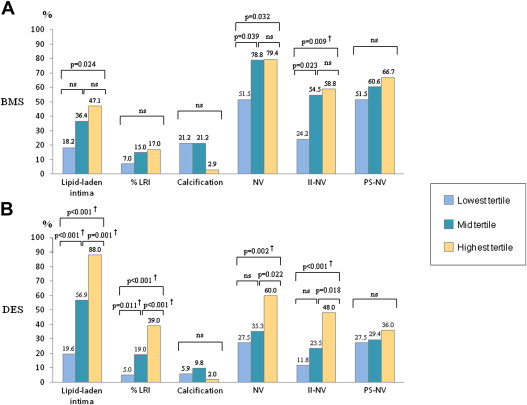
A total of 252 stents (100 BMSs and 152 DESs) were analyzed. NA was detected in 121 stents (48.0%): 37 BMSs (37.0%) and 84 DESs (55.3%). Mean and maximum NITs were significantly greater in stents with NA than those without NA ( Table 2 ). OCT analysis of incidence and characteristics of NA according to tertiles of mean NIT is shown in Figure 2 and Table 3 . In the BMS group, the prevalence of lipid-laden intima was progressively greater across the tertiles, with a significant difference between the lowest and the highest tertiles ( Figure 2 ). The percentage of LRI and the prevalence of calcification were not different between the tertiles (p = 0.124 and p = 0.053, respectively). NV, in particular with intra-intima location, was more frequent in the mid and highest tertiles than that in the lowest tertile. The prevalence of TCFA-like intima, disrupted intima, and thrombus was not different among the tertiles ( Table 3 ). In the DES group, the prevalence of lipid-laden intima and the percentage of LRI were progressively greater across the tertiles ( Figure 2 ), whereas no significant difference was observed in the prevalence of calcification (p = 0.252). The prevalence of NV, particularly with intra-intima location, TCFA-like intima, disrupted intima, and thrombus, was different among the tertiles, with significantly higher values in the highest tertile ( Table 3 ). Inter- and intraobserver agreements were k = 0.90 and k = 0.94 for lipid-laden intima and k = 0.92 and k = 0.93 for in-stent calcification, respectively.
| Variable | Lowest Tertile | Mid Tertile | Highest Tertile | p | |||
|---|---|---|---|---|---|---|---|
| Pearson Chi-Square | Lowest vs Mid Tertile | Mid vs Highest Tertile | Lowest vs Highest Tertile | ||||
| BMSs | 33 | 33 | 34 | ||||
| TCFA-like intima | 3 (9) | 6 (18) | 3 (9) | 0.410 | 0.473 | 0.444 | 0.697 |
| Disrupted intima | 1 (3) | 3 (9) | 4 (12) | 0.403 | 0.606 | 0.967 | 0.371 |
| Thrombus | 0 | 2 (6) | 5 (15) | 0.060 | 0.473 | 0.449 | 0.068 |
| DESs | 51 | 51 | 50 | ||||
| TCFA-like intima | 1 (2) | 9 (18) | 12 (24) | 0.005 | 0.020 | 0.588 | 0.003 ∗ |
| Disrupted intima | 1 (2) | 1 (2) | 9 (18) | 0.002 | 0.475 | 0.018 | 0.018 |
| Thrombus | 0 | 6 (12) | 10 (20) | 0.004 | 0.035 | 0.389 | 0.002 ∗ |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


