Coronary vessel distensibility is reduced with atherosclerosis and normal aging, but direct measurements have historically required invasive measurements at cardiac catheterization. Therefore, we sought to assess coronary artery distensibility noninvasively using 3.0 Telsa coronary magnetic resonance imaging (MRI) and to test the hypothesis that this noninvasive technique can detect differences in coronary distensibility between healthy subjects and those with coronary artery disease (CAD). A total of 38 healthy, adult subjects (23 men, mean age 31 ± 10 years) and 21 patients with CAD, diagnosed using x-ray angiography (11 men, mean age 57 ± 6 years) were studied using a commercial whole-body MRI system. In each subject, the proximal segment of a coronary artery was imaged for the cross-sectional area measurements using cine spiral MRI. The distensibility (mm Hg −1 × 10 3 ) was determined as (end-systolic lumen area − end-diastolic lumen area)/(pulse pressure × end-diastolic lumen area). The pulse pressure was calculated as the difference between the systolic and diastolic brachial blood pressure. A total of 34 healthy subjects and 19 patients had adequate image quality for coronary area measurements. Coronary artery distensibility was significantly greater in the healthy subjects than in those with CAD (mean ± SD 2.4 ± 1.7 mm Hg −1 × 10 3 vs 1.1 ± 1.1 mm Hg −1 × 10 3 , respectively, p = 0.007; median 2.2 vs 0.9 mm Hg −1 × 10 3 ). In a subgroup of 10 patients with CAD, we found a significant correlation between the coronary artery distensibility measurements assessed using MRI and x-ray coronary angiography (R = 0.65, p = 0.003). In a group of 10 healthy subjects, the repeated distensibility measurements demonstrated a significant correlation (R = 0.80, p = 0.006). In conclusion, 3.0-Tesla MRI, a reproducible noninvasive method to assess human coronary artery vessel wall distensibility, is able to detect significant differences in distensibility between healthy subjects and those with CAD.
Magnetic resonance imaging (MRI) offers the opportunity to noninvasively measure local vessel wall abnormalities and is not limited to the assessment of superficial vessels. Moreover, MRI can provide extensive coverage of large arteries with high accuracy and reproducibility. It has been used to characterize atherosclerotic plaques in the aorta and to measure the response to therapy at multiple vascular sites. In addition to structural information, MRI can acquire dynamic, physiologic data within the same vessels. High-field (3.0 Tesla [T]) MRI offers the advantage of high temporal and/or spatial resolution that can be used to quantify functional and morphologic alterations required to noninvasively measure in vivo coronary arterial distensibility. We sought to test the hypothesis that coronary artery distensibility can be quantified noninvasively using 3.0 T coronary MRI and that differences in distensibility can be detected between healthy subjects and those with coronary artery disease (CAD).
Methods
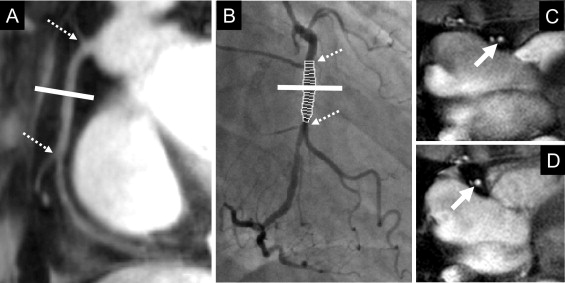
A total of 38 healthy, adult subjects (23 men, mean age 31 ± 10 years) and 21 patients with x-ray angiographically documented CAD (11 men, mean age 57 ± 6 years) were studied. Healthy subjects were defined as those without a history of CAD and the absence of traditional CAD risk factors, other than male gender. The patients with CAD were defined by the presence of coronary stenosis of ≥50% on previous coronary x-ray angiograms, a documented history of myocardial infarction, or a history of coronary revascularization, including percutaneous coronary intervention or coronary artery bypass grafting. The institutional review board at Johns Hopkins University (Baltimore, Maryland) approved the study protocol, and all participants provided written informed consent. The coronary MRI examination was performed in the morning, with the subjects in a fasting state and before the administration of prescribed vasoactive medications in patients. No contrast agent was used. Imaging was performed with the subjects in the prone position, and the distensibility measurements were obtained after ≥20 minutes of rest in the magnet. The peripheral blood pressure was recorded. The total imaging time was approximately 25 to 30 minutes. A commercial human 3.0 T MRI scanner (Achieva 3.0 T, Philips, Best, The Netherlands) with a 6-element cardiac coil for signal reception was used. Scout scans were performed to determine the 3-dimensional course of the proximal coronary arteries. Magnetic resonance angiography of the right coronary artery (RCA), left anterior descending artery (LAD), and/or left circumflex artery was performed using a navigator-gated, free-breathing, and electrocardiographically triggered, T 2 -weighted prepared, 3-dimensional, segmented k-space, gradient-echo imaging sequence. In each subject, the proximal segment of the coronary artery best identified on the scout images (RCA and/or LAD and/or left circumflex artery) was then imaged in cross section using cine spiral MRI for area measurements ( Figure 1 ). In the patients with CAD, the cross-sectional imaging plane was located in a region ≥2 cm proximal to the location of significant stenosis. The imaging plane for the cross-sectional area measurements was localized in a proximal arterial segment that was straight for a distance of ≥2 cm. Vein grafts were not included in the analysis. The MRI parameters were an echo time of 1.5 ms, radiofrequency excitation angle of 20°, spectral spatial excitation, breath-hold duration of ∼14 to 24 seconds, acquisition window of 10 ms, repetition time of 14 ms, 21 spiral interleaves, and spatial resolution (acquired/reconstructed) of 0.89 × 0.89 × 8.00 mm 3 /0.69 × 0.69 × 8.00 mm 3 . In a subgroup of 10 patients with CAD, clinically indicated invasive x-ray coronary angiography was performed within 6 months of MRI using a standard Judkins technique. The hemodynamics were monitored continuously throughout the procedure, and the pulse pressure was measured during coronary artery imaging. Coronary angiography was performed in multiple projections to obtain the optimum images for quantitative analysis. The coronary angiographic images were recorded in the universal Digital Imaging and Communications in Medicine format and forwarded to the Angiographic Core Laboratory for blinded analysis. Cross-sectional areas of the proximal coronary artery segments of 10 randomly selected subjects (7 healthy adults and 3 with CAD) were measured by 2 independent observers (S.K. and A.H.) and by 1 observer (A.H.) at 2 separate times. These data were used in the assessment of interobserver and intraobserver variability. Furthermore, 10 of our healthy subjects underwent 2 coronary MRI examinations to test for repeatability. After the first examination, the study subjects were removed from the scanner. After approximately 20 minutes outside the scanner, the subjects were then repositioned, and the complete examination was repeated. The coronary images were analyzed for absolute cross-sectional area changes using the full-width half-maximum criteria (cine version 3.15.17, GE, Milwaukee, Wisconsin). The images were magnified threefold, and a circular region of interest was manually traced around the coronary artery. The computer algorithm then automatically measured the cross-sectional coronary area using full-width half-maximum criteria. The distensibility (mm Hg −1 × 10 3 ) was determined as follows: (end-systolic lumen area − end-diastolic lumen area)/(pulse pressure × end-diastolic lumen area). The pulse pressure was calculated as the difference between the systolic and diastolic blood pressure. End-systole was defined as the period that began before the onset of left ventricular relaxation. End-diastole was defined as the period that began after the largest left ventricular volume was reached. For analysis of invasive coronary artery angiography, validated postprocessing software for computer-assisted quantitative coronary angiography was used (CAAS, version 2.0.1, Pie Medical Imaging BV, Maastricht, The Netherlands). The luminal diameter of the major epicardial coronary artery segment was quantified at end-systole and end-diastole in the cardiac cycle using quantitative angiography by an experienced interventionalist (J.M. or J.T.), who was unaware of the results of the MRI. Luminal diameter measurements were acquired at end-systole and end-diastole, as defined using electrocardiography. After the analysis, the MRI and invasive angiographic images were matched using the distances from the anatomic fiducial markers, such as the ostia or side branches, to ensure corresponding segments were measured by the 2 modalities. The Statistical Package for Social Sciences, version 12.0 for Windows (SPSS, Chicago, Illinois) was used for all statistical analyses. The data are expressed as the mean value ± SD. The study characteristics were compared using the Wilcoxon signed rank test. A nonparametric statistical test (Mann-Whitney U test) was used to compare the coronary cross-sectional area measurements and distensibility between the healthy subjects and those with CAD. Student’s paired t test was used to compare the invasive and MRI measurements in those patients with CAD who had undergone both modalities. To test for intra- and interobserver variability and repeatability, the data were analyzed using Pearson’s correlation coefficient and Bland-Altman agreement analysis. p Values for Bland-Altman were derived from Pitman’s test of difference in variance. Statistical significance was defined as a 2-tailed p value of <0.05.
Results
The image quality was adequate for coronary artery measurements in 34 (90%) of 38 healthy subjects and 19 (90%) of 21 patients with CAD. Of the 4 excluded healthy subjects, 1 each was excluded because of a broken coil, nondiagnostic image quality owing to excessive subject motion, shoulder pain, and breath-holding problems. Of the 2 excluded patients with CAD, 1 each was excluded because of claustrophobia and nondiagnostic image quality due to the body habitus. In healthy subjects, 54 coronary artery segments (32 RCA and 22 LAD) and in patients with CAD, 31 coronary artery segments (20 RCA, 10 LAD, and 1 left circumflex artery) were evaluable for analysis. The baseline characteristics of the study population are listed in Table 1 . The mean systolic and diastolic blood pressure in the healthy subjects was significantly lower than that in the patients with CAD (systolic pressure 121 ± 15 vs 141 ± 14 mm Hg, respectively, p <0.001) and (diastolic pressure 59 ± 10 vs 73 ± 13 mm Hg for diastolic pressure, respectively, p <0.001). The mean pulse pressure in the healthy adults (62 ± 10 mm Hg) was also significantly lower than that in the patients with CAD (68 ± 10 mm Hg, p <0.05). At end-systole, the luminal area was 11.6 ± 3.2 mm 2 in the healthy subjects and 14.3 ± 4.2 mm 2 in those with CAD (p <0.01). The luminal area during end-diastole was 10.3 ± 2.5 mm 2 for healthy subjects and 13.5 ± 3.9 mm 2 for those with CAD (p <0.001). The coronary vessel area change between end-systole and end-diastole was significantly greater in the healthy subjects than in those with CAD (13.5 ± 15.8% vs 5.9 ± 8.9%, respectively, p <0.01). The coronary artery distensibility was significantly greater in the healthy subjects than in those with CAD (2.4 ± 1.7 vs 1.1 ± 1.1 mm Hg −1 × 10 3 , respectively, p = 0.007; median 2.2 vs 0.8 mm Hg −1 × 10 3 ; Figure 2 ). The distensibility measurements, as well as the correlations between the corresponding coronary MRI and invasive x-ray coronary angiographic distensibility measures in a subgroup of 10 CAD patients, including 19 coronary artery segments, are listed in Table 2 . At end-systole, the luminal area was 13.8 ± 4.1 mm 2 using MRI and 10.0 ± 4.9 mm 2 using x-ray angiography (p = 0.002). The luminal area during end-diastole was 13.0 ± 3.4 mm 2 using MRI and 9.7 ± 4.8 mm 2 using invasive coronary angiography for the patients with CAD (p = 0.005). The mean pulse pressure measured at MRI in those with CAD (71 ± 10 mm Hg) was significantly greater than that at x-ray angiography (51 ± 11 mm Hg, p <0.001), likely owing to the sedation and nitroglycerin administered before invasive angiography. The average coronary artery luminal diameter stenosis assessed by x-ray coronary angiography was 18 ± 13%. We found no significant difference in the distensibility measurements between the 2 modalities (p = 0.62; Figure 3 ). A significant correlation was found between the coronary artery distensibility measurements assessed with MRI and invasive x-ray coronary angiography (y = 0.73x + 0.05, R 2 = 0.42, p = 0.003; Figure 3 ). Intraobserver and interobserver analyses for measurement of coronary distensibility were performed in 10 subjects (7 healthy subjects and 3 patients with CAD), including 16 proximal coronary artery segments. We found a high correlation for distensibility measurements performed by 1 observer (R = 0.91, p <0.001) or between 2 observers (R = 0.76, p = 0.001; Figure 4 ). In 10 healthy subjects, including 10 proximal coronary artery segments, the repeatability measurements for distensibility between 2 different MRI examinations performed on the same day demonstrated a significant correlation (R = 0.80, p = 0.006; Figure 4 ).
| Characteristic | Healthy Subjects (n = 38) | Patients With CAD (n = 21) | p Value |
|---|---|---|---|
| Age (years) | 31 ± 10 | 57 ± 6 | <0.001 |
| Men | 23 (61%) | 11 (52%) | 0.002 |
| Family history of coronary artery disease | 0 | 6 (29%) | |
| Previous myocardial infarction | 0 | 4 (19%) | |
| Percutaneous coronary intervention/stent | 0 | 10 (48%) | |
| Coronary artery bypass grafting | 0 | 3 (14%) | |
| Smoking | 0 | 8 (38%) | 0.005 |
| Dyslipidemia | 0 | 18 (86%) | <0.001 |
| Diabetes mellitus | 0 | 8 (38%) | 0.005 |
| Arterial hypertension | 0 | 15 (71%) | <0.001 |
| Target coronary artery | |||
| Left | 22 (41%) | 11 (35%) | |
| Right | 32 (59%) | 20 (65%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


