16 Coronary Artery Disease
 Pathoanatomical and Pathophysiological Basics
Pathoanatomical and Pathophysiological Basics
Coronary artery disease is among the most common human diseases and represents currently the main indication for diagnostic cardiac catheterization. Clinically the disease manifests itself as
 Stable angina
Stable angina
 Acute coronary syndrome: ST elevation myocardial infarction, non–ST-elevation myocardial infarction, unstable angina
Acute coronary syndrome: ST elevation myocardial infarction, non–ST-elevation myocardial infarction, unstable angina
 Heart failure
Heart failure
 Cardiac arrhythmias
Cardiac arrhythmias
 Sudden cardiac death
Sudden cardiac death
Pathoanatomically the disease is based in the majority of cases upon an obstructive coronary sclerosis of the large epicardial vessels, as a manifestation of atherosclerosis of the coronary arteries. In the early stages of the disease there are usually no clinical symptoms, even though the vascular walls already have endothelial dysfunction and lipid deposits. The acute coronary syndrome is caused by the rupture of an atherosclerotic plaque with subsequent thrombotic complete or incomplete occlusion of the vessel. Functional stenoses in the context of a vasospastic angina can also cause angina.
Pathophysiologically there is a mismatch between oxygen demand and oxygen supply of the dependent myocardium.
 Goals
Goals
The main objective of cardiac catheterization in coronary artery disease is the evaluation of the degree and the morphology of coronary stenoses, which represent the most important morphological factors affecting oxygen supply. Further objectives are to evaluate potential collaterals and to relate the coronary findings to regional and global left ventricular systolic function. In addition, the coronary morphology has to be put into context with the results of functional tests and, if available, noninvasive imaging results.
Coronary Insufficiency
Coronary insufficiency as a result of reduced oxygen supply is influenced by
 Morphological changes of the coronary arteries:
Morphological changes of the coronary arteries:
– Coronary stenosis
– Collateral formation
– Vasoconstriction (dynamic obstruction, vasospastic angina)
– Coronary compression with coronary anomalies
– Shunts through coronary fistulas
 Functional factors:
Functional factors:
– Perfusion pressure
– Diastolic aortic pressure
– Diastolic ventricular pressure
– Oxygen content in the arterial blood (severe anemia)
– Oxygen saturation
– Blood viscosity
In contrast, increased oxygen consumption is found in
 Increased heart weight (hypertrophy)
Increased heart weight (hypertrophy)
 Increased heart rate
Increased heart rate
 Increased contractility
Increased contractility
 Increased myocardial wall tension (hypertrophy, dilatation)
Increased myocardial wall tension (hypertrophy, dilatation)
Thus, the causes of coronary insufficiency are numerous. In addition, there are important processes regarding metabolic regulation of coronary perfusion and activation of the coagulation system, which play central roles especially during an acute coronary syndrome.
Coronary Stenosis
The progressive narrowing of the coronary arteries causes a progressive reduction in the poststenotic perfusion pressure. Via compensatory dilatation of the downstream arterioles with a corresponding reduction in poststenotic vascular resistance the coronary perfusion can nevertheless be maintained in the normal range up to a lumen narrowing of ~50 %. This compensatory mechanism to increase coronary perfusion is referred to as coronary reserve and permits in healthy individuals (with pharmacological dilatation of the arterioles) a four-fold increase in coronary perfusion.
However, in patients with CAD the maximally possible increase in coronary perfusion drops continuously starting at a lumen narrowing of 50 %. For a lumen narrowing of 75 % and higher, a halving of the coronary reserve must be assumed, which can usually be detected in stress tests as ischemia. For a lumen narrowing of > 90 %, the perfusion is already impaired at rest.
Clinically, the diagnosis of coronary stenoses together with ventricular function, clinical symptoms and demonstration of ischemia form the basis for the management of individual patients with CAD. Important factors that influence the decision to proceed with PCI versus surgical revascularization versus medical therapy include the number of vessels involved, the degree of stenosis, the morphology and location of the stenoses, and the patient’s general clinical status (comorbidities, etc.).
Assessment of the Degree of the Stenosis
During interpretation of the coronary angiogram the degree of stenosis is assessed as percent diameter reduction compared with the diameter of adjacent, nondiseased vessel segments.
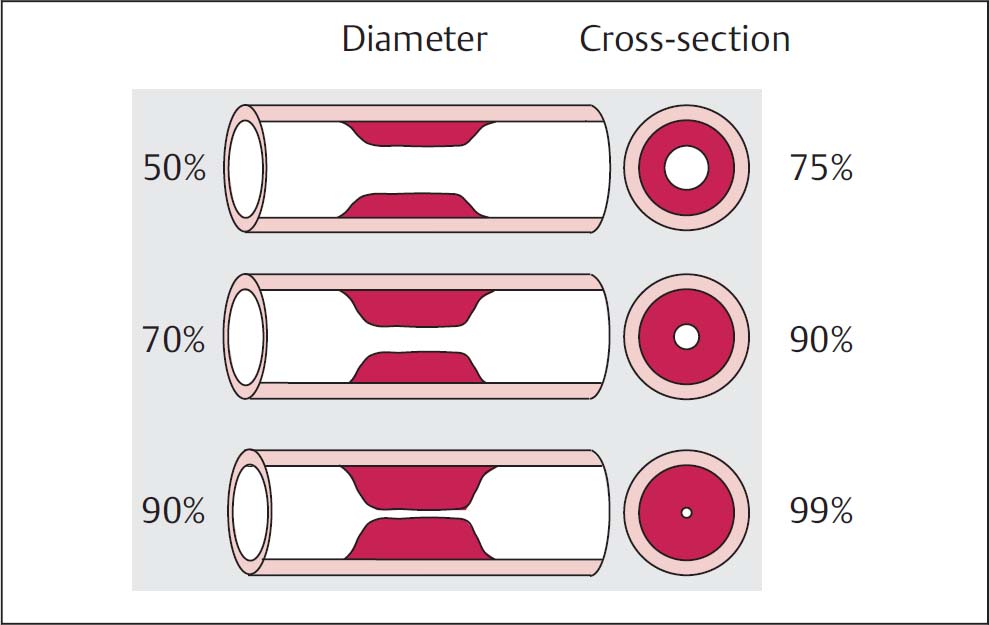
 Thedegree of stenosis as a reduction in diameter is not to be equated with the degree of stenosis assessed as lumen narrowing or reduction in cross-section, as Fig. 16.1 makes evident.
Thedegree of stenosis as a reduction in diameter is not to be equated with the degree of stenosis assessed as lumen narrowing or reduction in cross-section, as Fig. 16.1 makes evident.
An angiographic stenosis of 50 % corresponds to a lumen narrowing of 75 % and thus is a stenosis that begins to be hemodynamically relevant. A 75 % stenosis can be equated to a 95 % lumen narrowing. A stenosis of 90 % corresponds to a lumen of ~1 % and markedly delayed blood flow. These two concepts should always be properly differentiated.
In coronary angiography the degree of stenosis is in general assessed semiquantitatively by the operator. While this requires some practice and experience, the visual assessment of the degree of stenosis is a relatively accurate method, which shows results similar to computer-based quantitative stenosis measurement. A precise quantification of the stenosis (e.g., 56 % or 92 %) is neither possible nor clinically meaningful. In contrast, the following categorization of lesion severity—which is adapted from a proposal by the American Heart Association—is of practical relevance:
Fig. 16.1 Assessment of the degree of stenosis for a concentric stenosis. Comparison of the reduction of the diameter with the reduction of the cross-section.
 ≤ 25 %: contour irregularities or diffuse, nonobstructive coronary artery sclerosis
≤ 25 %: contour irregularities or diffuse, nonobstructive coronary artery sclerosis
 25 to 50 %: mild stenosis
25 to 50 %: mild stenosis
 50 to 75 %: moderate stenosis
50 to 75 %: moderate stenosis
 > 75 %: severe stenosis
> 75 %: severe stenosis
 100 %: complete occlusion
100 %: complete occlusion
The classification is based on the diameter of the stenosis and not on the reduction in the vessel cross-section/lumen.
Sources of error: prerequisite for the correct recognition and evaluation of coronary stenoses is the proper imaging of all segments of the coronary arteries in several (at least two) projection planes. The evaluation of the lesion severity is done exclusively in diastole.
Possible sources of error when assessing the lesion severity are
 Overlap of the stenotic vessel segment with a nondiseased vessel
Overlap of the stenotic vessel segment with a nondiseased vessel
 Imaging of a stenotic vessel segment in a foreshortened projection with overestimation of the lesion severity
Imaging of a stenotic vessel segment in a foreshortened projection with overestimation of the lesion severity
 Opacification too weak with misjudgment of the stenosis severity due to flow
Opacification too weak with misjudgment of the stenosis severity due to flow
 Eccentric stenosis with inadequate projection resulting in underestimation of lesion severity (Fig. 16.2)
Eccentric stenosis with inadequate projection resulting in underestimation of lesion severity (Fig. 16.2)
 Vasospasm instead of fixed coronary stenosis, especially in the area of the RCA ostium
Vasospasm instead of fixed coronary stenosis, especially in the area of the RCA ostium
 Coronary anomaly, misinterpreted as vessel occlusion
Coronary anomaly, misinterpreted as vessel occlusion
 Missed ostial stenosis because the catheter is too deeply engaged
Missed ostial stenosis because the catheter is too deeply engaged
 Misjudgment of the lesion severity due to poststenotic dilatation or ectatic forms of coronary artery disease
Misjudgment of the lesion severity due to poststenotic dilatation or ectatic forms of coronary artery disease
 Problems of digital image processing in the areas of vessel overlap or in the area of bifurcations (edge phenomenon)
Problems of digital image processing in the areas of vessel overlap or in the area of bifurcations (edge phenomenon)
 Incorrect assessment by the individual operator
Incorrect assessment by the individual operator
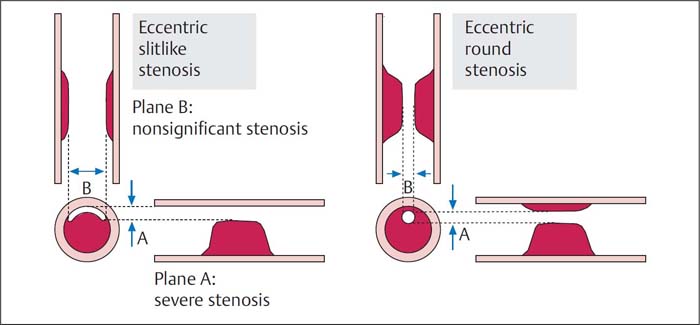
Fig. 16.2 Assessment of lesion severity with an eccentric stenosis. In contrast to eccentric stenoses with circular remaining lumen, the lumen severity with slitlike eccentric stenosis differs depending on projection.
Quantitative coronary angiography (QCA). The interobserver variability in the assessment of lesion severity provides a frequent criticism of the visual evaluation of coronary stenoses. Nevertheless, for most clinical problems this method is the most practical, especially considering that patient management does not depend solely on lesion severity but is also influenced by clinical symptoms and the results of functional tests.
For scientific studies, e.g., for quantification of the therapeutic success and the recurrence rate after a specific interventional procedure, or for the evaluation of plaque regression with lipid-lowering therapy, the purely visual assessment of degree of stenosis is insufficient.
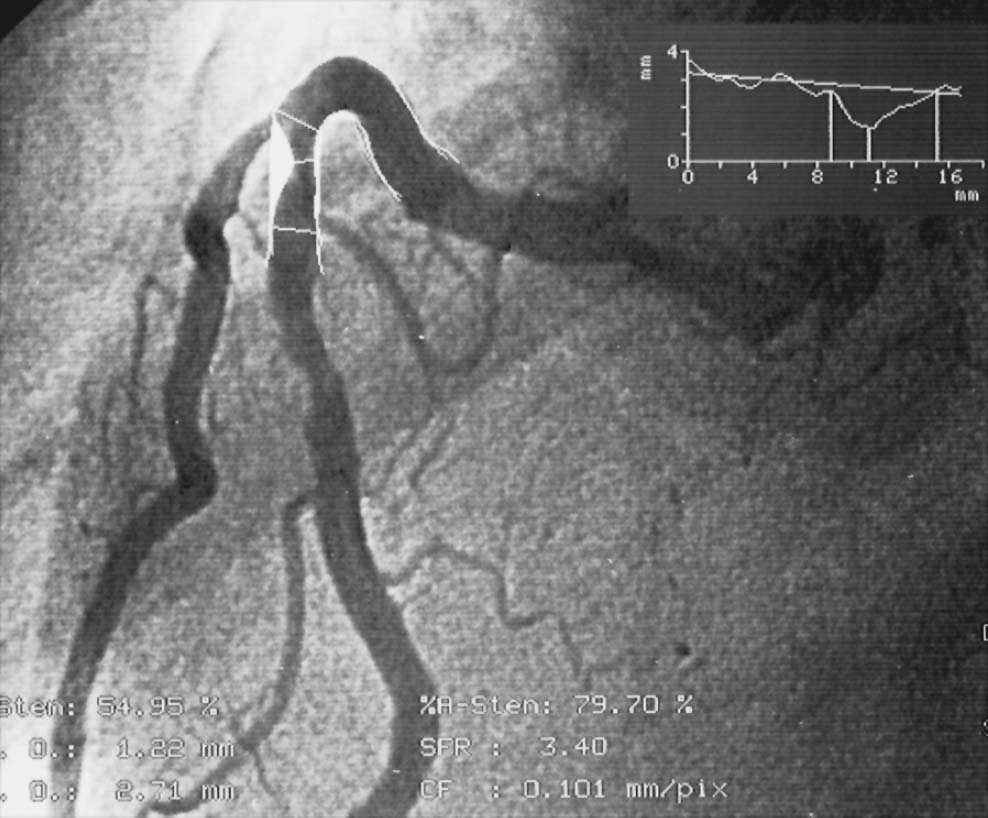
The method of quantitative coronary angiography is based upon automatic contour detection of a contrast medium-filled vessel segment using grayscale changes at the border of the vessel. The analyst determines the proximal and distal end of the vessel segment to be examined, corrects the displayed vessel contour, and determines the reference cross-diameter in a nondiseased segment (Fig. 16.3). Calibration is done using the positioned coronary catheter.
The “on-line” use of QCA systems is possible with digital catheter systems. It can be particularly useful for coronary interventions and help in the selection of the size of balloon and stent diameter and also for evaluating therapeutic success.
In contrast, the scientific assessment of coronary angiographic findings is based on the digital evaluation of the coronary angiography dataset.
Fig. 16.3 Quantitative coronary angiography (QCA). On-line evaluation of a moderate proximal stenosis of the right coronary artery.
The main problem when repeated coronary angiographies are compared is guaranteeing identical conditions during the examination. Not only must the examination be performed on the same catheterization system, but also the following must be the same:
 Angle adjustment
Angle adjustment
 Image intensifier–tube distance
Image intensifier–tube distance
 Table height
Table height
 Position of the patient on the table
Position of the patient on the table
 Inspiration depth
Inspiration depth
 Magnification
Magnification
 Size of the calibration catheter
Size of the calibration catheter
 Medication
Medication
 Contrast medium
Contrast medium
In addition, the vessel segment to be examined must be visualized with the same center, and a possible dynamic component of the coronary stenosis has to be eliminated by prior administration of nitrates.
Coronary Flow
Especially in patients with unstable angina pectoris or acute myocardial infarction, classification of coronary flow according to the TIMI classification has proved useful. The classification was originally developed to evaluate the success of thrombolytic therapy.
 Grade 0: no perfusion
Grade 0: no perfusion
 Grade 1: penetration of the contrast medium at the stenosis/occlusion site, without complete opacification of the distal vessel segments
Grade 1: penetration of the contrast medium at the stenosis/occlusion site, without complete opacification of the distal vessel segments
 Grade 2: perfusion with delayed, but complete opacification of distal vessel segments
Grade 2: perfusion with delayed, but complete opacification of distal vessel segments
 Grade 3: prompt, complete perfusion of the vessels
Grade 3: prompt, complete perfusion of the vessels
It is obvious that coronary flow does not depend solely on the degree of stenosis but is also influenced by other factors such as vasospasm and thrombi. Even at rest severe coronary stenoses cause a marked impairment of the blood flow, which is characterized by a delayed opacification of the poststenotic vessel segments. Impaired flow, occasionally with stasis of the contrast medium, without high-grade stenosis is observed in the ectatic or dilative form of coronary atherosclerosis and is a possible starting point for local thrombus formation. In addition, contrast runoff can also be delayed in very large vein grafts.
If initially no severe stenosis can be detected but there is localized impaired flow, the coronary angiogram should be carefully reevaluated. If required, additional projections should be used in order not to miss a proximal stenosis, for example.
 To evaluate contrast flow, it is important that the patient does not perform a Valsalva maneuver during the injection. Otherwise the increased intrathoracic pressure and (in some cases substantial) increase in left ventricular diastolic pressure can impair the runoff of the contrast medium (misinterpreted as a “slow-flow” phenomenon).
To evaluate contrast flow, it is important that the patient does not perform a Valsalva maneuver during the injection. Otherwise the increased intrathoracic pressure and (in some cases substantial) increase in left ventricular diastolic pressure can impair the runoff of the contrast medium (misinterpreted as a “slow-flow” phenomenon).
Stenosis Morphology
Morphological evaluation of the coronary stenosis is important for
 Decisions regarding the therapy of coronary artery disease: PCI versus CABG versus medical therapy
Decisions regarding the therapy of coronary artery disease: PCI versus CABG versus medical therapy
 Risk assessment of a planned PCI
Risk assessment of a planned PCI
 The selection of the interventional procedure for planned PCI
The selection of the interventional procedure for planned PCI
The major morphological criteria are
 Length:
Length:
– Short (≤ 1 cm)
– Tubular (1 to 2 cm)
– Diffuse (> 2 cm)
 For the same degree of stenosis a long stenosis is hemodynamically more relevant than a short stenosis.
For the same degree of stenosis a long stenosis is hemodynamically more relevant than a short stenosis.
 Contour:
Contour:
– Smooth
– Irregular
 Location:
Location:
– Eccentric
– Concentric
– Side branch
– Ostial
 Calcification:
Calcification:
– None/little
– Marked
 In-stent restenosis:
In-stent restenosis:
– Focal
– Diffuse/proliferative The morphological criteria are part of the stenosis classification. These form the basis for therapeutic success and risk assessment of PCI with different stenosis morphologies. Location of the lesion, relation to side branches, the tortuosity of the vessel and the presence of thrombi are also considered (Table 16.1).
Table 16.1 Lesion morphology according to the criteria of AHA/ACC
Type A – Discrete (< 10 mm) – Concentric – Readily accessible – Nonangulated segment (< 45°) – Smooth contour – Little or no calcium – Less than totally occlusive – Not ostial in location – No major side branch – Absence of thrombus |
Type B – Tubular (10–20 mm length) – Eccentric – Moderate tortuosity of proximal segment – Moderately angulated (45–90°) – Irregular contour – Moderate to heavy calcification – Total occlusion < 3 months – Ostial stenosis – Bifurcation lesion requiring double guidewire – Some thrombus present |
B1 = 1 criterion fulfilled B2 = 2 or more criteria fulfilled |
Type C – Diffuse (> 20 mm length) – Excessive tortuosity of proximal segment) – Extremely angulated segment (> 90°) – Total occlusion > 3 months old – Inability to protect major side branches – Degenerated vein grafts with friable lesions |
C1 = 1 criterion fulfilled C2 = 2 or more criteria fulfilled |
 Justby reading the description of the findings it should be possible to get a detailed impression of the morphology of the stenosis.
Justby reading the description of the findings it should be possible to get a detailed impression of the morphology of the stenosis.
Relevance of the Location of the Stenosis
Ostial stenoses as well as stenoses in the proximal, middle, and distal thirds of the respective coronary artery are differentiated.
The more proximally a stenosis is located, the larger is the affected area of the myocardium and the clinical relevance of the coronary stenosis. An exception is the right coronary artery, the long proximal segment of which up to the crux of the heart does not give off branches to the left ventricle; thus, distal stenoses proximal to the crux frequently have the same clinical relevance as proximal stenoses.
As with the morphology of the lesion, the location and the number of severe lesions influence patient management.
The following are differentiated:
 Number of vessels diseased
Number of vessels diseased
 Stenosis location in the vessel and involvement of side branches
Stenosis location in the vessel and involvement of side branches
Number of vessels diseased. The classification depends on the detection of a stenosis > 50 % in the three-vessel system consisting of
 Left circumflex (LCX)
Left circumflex (LCX)
 Left anterior descending (LAD)
Left anterior descending (LAD)
 Right coronary artery (RCA)
Right coronary artery (RCA)
Cardiac Catheterization Report in CAD
In the cardiac catheterization report the angiographic findings should be described in such a way that colleagues can gain a detailed impression of the findings and their therapeutic consequences. The cardiac catheterization report should contain in addition to the standard information, which is described in Chapter 6, the following information:
 Brief cardiac history plus relevant comorbidities such as chronic kidney disease
Brief cardiac history plus relevant comorbidities such as chronic kidney disease
 Current clinical symptoms
Current clinical symptoms
 Result of cardiac functional tests For coronary findings:
Result of cardiac functional tests For coronary findings:
 Left or right dominance
Left or right dominance
 Number of vessels diseased
Number of vessels diseased
 Individual description of the left coronary main stem
Individual description of the left coronary main stem
 Individual description of the right and left coronary artery with description of the location of a stenosis:
Individual description of the right and left coronary artery with description of the location of a stenosis:
– Proximal, middle, distal third of the vessel
– Location of the stenosis in relation to significant side branches (proximal or distal to origins of septal, diagonal or marginal branches)
– Location of stenosis according to the segments of the AHA classification (Fig. 16.4)
 Description of stenosis morphology (AHA/ACC criteria)
Description of stenosis morphology (AHA/ACC criteria)
 For severe stenoses, description of the coronary flow
For severe stenoses, description of the coronary flow
 For occluded vessels, description of collateral circulation
For occluded vessels, description of collateral circulation
 Ventricular function: detailed description of wall motion abnormalities (normokinesia, hypokinesia, akinesia or dyskinesia); report EF
Ventricular function: detailed description of wall motion abnormalities (normokinesia, hypokinesia, akinesia or dyskinesia); report EF
 Hemodynamics, especially LVEDP
Hemodynamics, especially LVEDP
 Summary diagnosis
Summary diagnosis
 Treatment recommendation, if already possible: PCI, CABG or medical therapy
Treatment recommendation, if already possible: PCI, CABG or medical therapy
 Required additional tests (cardiac MRI, cardiac CT, exercise stress test, stress echocardiography, stress nuclear study, right heart catheterization, pressure wire, intravascular ultrasound (IVUS)
Required additional tests (cardiac MRI, cardiac CT, exercise stress test, stress echocardiography, stress nuclear study, right heart catheterization, pressure wire, intravascular ultrasound (IVUS)
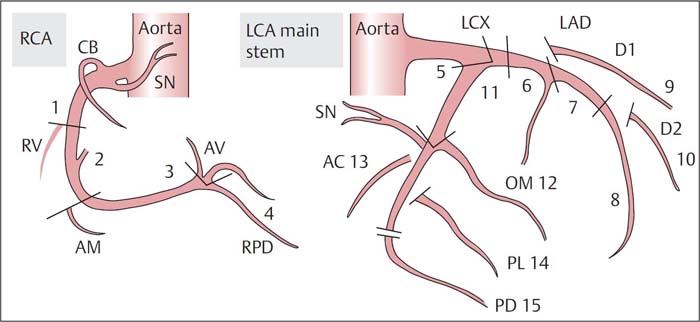
Fig. 16.4 Coronary segments according to the system proposed by the AHA.
AC, atrial circumflex branch;
AM, acute marginal branch;
AV, atrioventricular node;
CB, conus branch;
D1, first diagonal branch;
D2, second diagonal branch;
LAD, left anterior descending coronary artery;
LCA, left coronary artery;
LCX, left circumflex coronary artery;
OM, obtuse marginal branch;
PD, posterior descending branch;
PL, posterolateral branch;
RCA, right coronary artery;
RPD, right posterior descending branch;
RV, right ventricle;
SN, sinus node.
Location of the stenosis in the vessel. Regarding the left coronary artery, stenoses of the left main stem are associated with a poor prognosis and increased risk during cardiac catheterization.
Special problems for angiographic imaging can also be posed by stenoses at the ostium or the origin of the LAD or the LCX. This region is frequently difficult to assess due to overlap of the affected vessel segments, and therefore regularly requires additional (usually highly angulated) projections. The origins of the large side branches of LCX and LAD also have to be imaged separately.
Bifurcation stenoses and stenoses that are located so that a large side branch cannot be protected require special experience and techniques for PCI); they can be the reason for deciding against PCI and recommending CABG. PCIs in the RCA are more problematic if stenoses are located at the ostium or at the bifurcation at the crux of the heart, when the origins of the two main vessels, posterior left ventricular branch and PDA, are involved. A stenosis at the origin of the PDA can be assessed frequently only in an angulated LAO projection.
Collateral Circulation
The coronary arteries are not end arteries but are connected to each other by a network of precapillary collaterals. These vessels have a diameter of less than 0.2 mm and are usually not visible in the angiogram. The number and pattern of distribution are probably genetically determined. If a coronary artery is occluded or subtotally stenotic (> 95 %), the collateral can develop into larger vessels that are easily visible in the angiogram and which supply the distal segments of the occluded coronary artery and thus the corresponding myocardium with blood.
Significant determinants of collateral formation are repeated ischemic episodes, increased shear forces due to the higher perfusion pressure in the small preformed vessels and growth factors (VEGF, TGF-α, α-FGF, and others). It is also not obvious or predictable why some patients with vessel occlusion have collaterals and some do not.
As formation of collaterals is a time-dependent process, they can develop more easily in slowly progressive coronary stenosis than with a sudden occlusion of a ruptured atheromatous plaque.
If appropriate large collaterals are present, left ventricular function can remain unimpaired despite complete proximal occlusion of a coronary artery. The collaterals are thus able to provide sufficient perfusion at rest and under low stress. However, the coronary reserve is as expected significantly impaired: the maximal collateral perfusion with unimpaired left ventricular function is ~30 to 40 % of normal and usually leads to typical exercise-dependent angina in patients with the following constellation of findings:
 Proximal vessel occlusion
Proximal vessel occlusion
 Well-developed collateral circulation
Well-developed collateral circulation
 Normal ventricular function
Normal ventricular function
The following types of collaterals are differentiated:
1. Antegrade collaterals, also called bridging collaterals: connections between different segments of the same artery in the form of mostly relatively short vessels (enlarged vasa vasorum or adventitial vessels)
2. Intracoronary collaterals: connections between segments of the same coronary artery proximally and distally to the site of occlusion
3. Intercoronary collaterals: connections between different coronary arteries
The possible paths of the collateral circulation can be very variable and depend on the severity and location of the coronary stenoses, and on a person’s native propensity to form collaterals.
Frequent collateral connections are shown in Table 16.2.
Depending on the severity and extent of the coronary artery disease there can be several collateral circulations. The operator should pay attention to collateral vessels in every angiographic procedure. To make that possible, a sufficiently long image recording is required, as the collaterals frequently fill only in the late phase after contrast injection. In addition, the field of view must not be collimated too much, as otherwise those segments of a (contralateral) coronary artery that fill retrogradely via collaterals cannot be assessed.
A practical way to describe collateral circulation is the classification according to Rentrop:
 Collateral circulation grade 0: no collateral vessels visible
Collateral circulation grade 0: no collateral vessels visible
 Collateral circulation grade 1: opacification of the side branches of the stenotic or occluded coronary artery without imaging of the epicardial segments (e.g., with right coronary injection: retrograde opacification of the septal branches of the LAD)
Collateral circulation grade 1: opacification of the side branches of the stenotic or occluded coronary artery without imaging of the epicardial segments (e.g., with right coronary injection: retrograde opacification of the septal branches of the LAD)
 Collateral circulation grade 2: partial retrograde opacification of the epicardial segments of the stenotic or occluded coronary artery
Collateral circulation grade 2: partial retrograde opacification of the epicardial segments of the stenotic or occluded coronary artery
 Collateral circulation grade 3: complete retrograde opacification of the stenotic or occluded coronary artery up to the site of occlusion
Collateral circulation grade 3: complete retrograde opacification of the stenotic or occluded coronary artery up to the site of occlusion
Table 16.2 Frequent collateral connections
Collateral | Intracoronary | Intercoronary |
To the RCA | – From acute marginal or posterior left ventricular branch to distal RCA – From atrial branches to distal RCA | – From the posterolateral branches of the LCX to posterior left ventricular branch Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
 Quantitative coronary angiography does not permit a definitive assessment of the functional relevance of the respective coronary stenosis.
Quantitative coronary angiography does not permit a definitive assessment of the functional relevance of the respective coronary stenosis.