8 Coronary Angiography
 Goals
Goals
Coronary angiography is the gold standard for imaging the morphology of coronary arteries. The goal is the complete imaging of all epicardial coronary vessels including collaterals and, if required, coronary bypasses after coronary artery bypass graft (CABG) or coronary veins.
The examination facilitates therapeutic decisions in patients with known or suspected coronary artery disease. Coronary angiography cannot be evaluated in isolation, but is evaluated rather in context with symptomatic status and cardiac function tests (e.g., stress testing with or without perfusion scanning, stress echocardiography).
General Considerations
The following general rules are provided prior to discussion of the details of cardiac catheterization.
1. Cardiac catheterization involves primarily intellectual input and only secondarily technical–physical effort. The indication has to be correct and the examination has to planned appropriately. If problems occur there has to be an alternative plan. The catheter always has to be controlled by the operator and not the other way around. A broad knowledge of the equipment is indispensable.
2. There should be no “blind” catheter maneuvers—never work without fluoroscopy.
3. Never work against resistance. If a catheter cannot be moved freely, a cause has to be identified; always consider dissections or perforations.
4. Allow sufficient backflow of blood to flush every newly introduced catheter before connecting and injecting. When the catheter is advanced, air, atheromas, or displaced thrombi can enter the catheter, especially with large guiding catheters. Before engaging the coronary arteries, a test injection should be done in the aorta.
5. Image and evaluate every finding before continuing the examination. Image especially unexpected, unusual findings, and anomalies. The same is true for complications.
6. Do not take anything for granted. Every examination is unique. The operator should always check materials prepared by others (e.g., air in pressure or contrast medium tubing).
7. Always examine carefully and never with force. This is especially true for complex interventions such as valvuloplasties, transseptal procedures, etc.
8. Pain indicates a problem that must be investigated immediately.
9. The invasive examination or procedure should only be completed when all questions that can be answered have been answered.
 Indications
Indications
Indications for coronary angiography are summarized in Table 8.1.
Coronary Artery Disease
Coronary angiography is indicated to plan treatment in patients with clinically proven or suspected coronary artery disease (CAD). Without knowledge of the coronary morphology it is not possible to decide which of the following therapies is most appropriate for a patient:
 Percutaneous coronary intervention
Percutaneous coronary intervention
 Surgical revascularization
Surgical revascularization
 Medical therapy
Medical therapy
Alternatively, cardiac MRI and especially cardiac CT are evolving as noninvasive methods for imaging the morphology of the coronary arteries (see p. 66).
Urgency, the timing of the examination, and the requirement for pretests depend on the clinical manifestation of the CAD and on circumstances that can increase the risk of coronary angiography.
Table 8.1 Possible indications for coronary angiography
– Coronary artery disease – Stable angina on exertion (particularly if lifestylelimiting) – Patients with high risk characteristics and ambiguous noninvasive test results – Unstable angina – Acute myocardial infarction (STEMI and NSTEMI) – Post infarction – To exclude CAD – Evaluation of coronary arteries before surgery/intervention of congenital or acquired valvular disease – Control coronary angiography after PCI in patients at risk – Ventricular tachyarrhythmias and survived sudden cardiac death of unknown etiology – Heart failure of unknown origin |
In patients with stable angina functional cardiac tests should already have been performed before coronary angiography. In addition, appropriate measures should be taken to reduce the risk of the examination, such as improving renal function or appropriately pretreating hyperthyroidism (see Chapter 6), addressing coagulopathies, and if necessary pretreating with platelet aggregation inhibitors, etc.
For unstable angina coronary angiography within 48 hours is recommended so that the necessary therapeutic steps (e.g., percutaneous coronary intervention [PCI] or cardiac surgery) can occur in a timely manner. Initially, medical stabilization is attempted, but this should not significantly delay the angiography.
 If the patient’s complaints are refractory to treatment, then perform immediate coronary angiography.
If the patient’s complaints are refractory to treatment, then perform immediate coronary angiography.
In patients with acute myocardial infarction, coronary angiography with subsequent intervention is the treatment of choice. Coronary angiography with subsequent intervention should also be pursued in the case of contraindications for thrombolysis or if patients after thrombolysis continue to have complaints or signs of ischemia.
During the postinfarction phase, after thrombolysis but also in medically treated patients, coronary angiography can be done electively if the patient is asymptomatic. Prior to catheterization, patients can be mobilized and functional testing can be completed.
After surgical revascularization, coronary angiography is indicated if angina persists unexpectedly or if symptoms recur after an initial asymptomatic interval. It is important to know the presurgical coronary status and the surgical details (surgical report).
In the past, there have been restenosis rates of up to 30 % within 3 to 6 months. This rate is much lower with current drug eluting stents. Current guidelines of professional societies do not recommend routine surveillance coronary angiography.
These are only recommended in patients with high risk interventions, especially after left main PCI. Clinical practice is quite variable.
Further Indications
Exclusion of coronary artery disease. A frequent dilemma is the issue of coronary angiography in patients with recurrent atypical chest pain and in patients with unremarkable findings on functional testing. This “relative” indication for coronary angiography to exclude CAD should be met with hesitation and with special consideration of the individual risk of the examination. Nevertheless, for some patients it can be better to rule out definitively CAD, as compared to months or years of repeated hospitalizations and stress tests, without providing a clear-cut finding or an unambiguous differential diagnosis. Alternatively, an indication for cardiac CT may be considered here.
Cardiac catheterization before surgery of valvular and/or congenital heart disease. In many patients with congenital or acquired valvular or congenital heart disease echocardiography is sufficient to determine the need for surgery, and cardiac catheterization is not necessarily required. However, coronary angiography is recommended in all patients aged 40 years or older because advanced atherosclerosis may not always be detected with functional testing or cause symptoms. In addition, cardiac symptoms due to the valvular or congenital heart disease can obscure coexisting coronary symptoms. In younger patients the indication for coronary angiography should be discussed with the cardiac surgeon. When there are risk factors for CAD, we recommend a low threshold for performing coronary angiography.
Coronary angiography before surgery on the thoracic aorta (aneurysm, dissection). Aortic dissection or aneurysm is diagnosed with noninvasive imaging studies (CT, MRI, TEE [transesophageal echocardiography]). As aortic disease of atherosclerotic etiology frequently coincides with CAD, there is always the question whether preoperative coronary angiography should be performed. With acute type A dissection, routine coronary angiography before emergency surgery is not indicated because of the risk and the time delay. The decision should be made on a case-by-case basis in consultation with the surgeon.
In these patients we always perform the CT as an ECG-triggered CT-angiography (cardiac CT) so as to also achieve adequate imaging of the coronaries.
Ventricular tachyarrhythmias of unknown etiology. CAD is frequently the cause of ventricular tachyarrhythmias, and the status of the coronary arteries determines subsequent management.
Heart failure of unknown etiology. In many cases heart failure with a dilated, poorly contracting left ventricle is not due to an idiopathic dilated cardiomyopathy, but rather to CAD. Differentiation should be done in a timely manner in order not to delay possible therapeutic options (CABG, PCI). Noninvasive methodologies frequently cannot provide this differentiation, so that coronary angiography should be considered after assessing potential risks.
In addition, when recording the venous contrast phase, coronary angiography provides sufficient imaging of the coronary veins for potential resynchronization therapy.
 Materials
Materials
Instruments for arterial puncture
 Puncture needle
Puncture needle
 Arterial sheath (4F, 5F, 6F, or 7F)
Arterial sheath (4F, 5F, 6F, or 7F)
 Guidewire 0.035 in., length 145 cm
Guidewire 0.035 in., length 145 cm
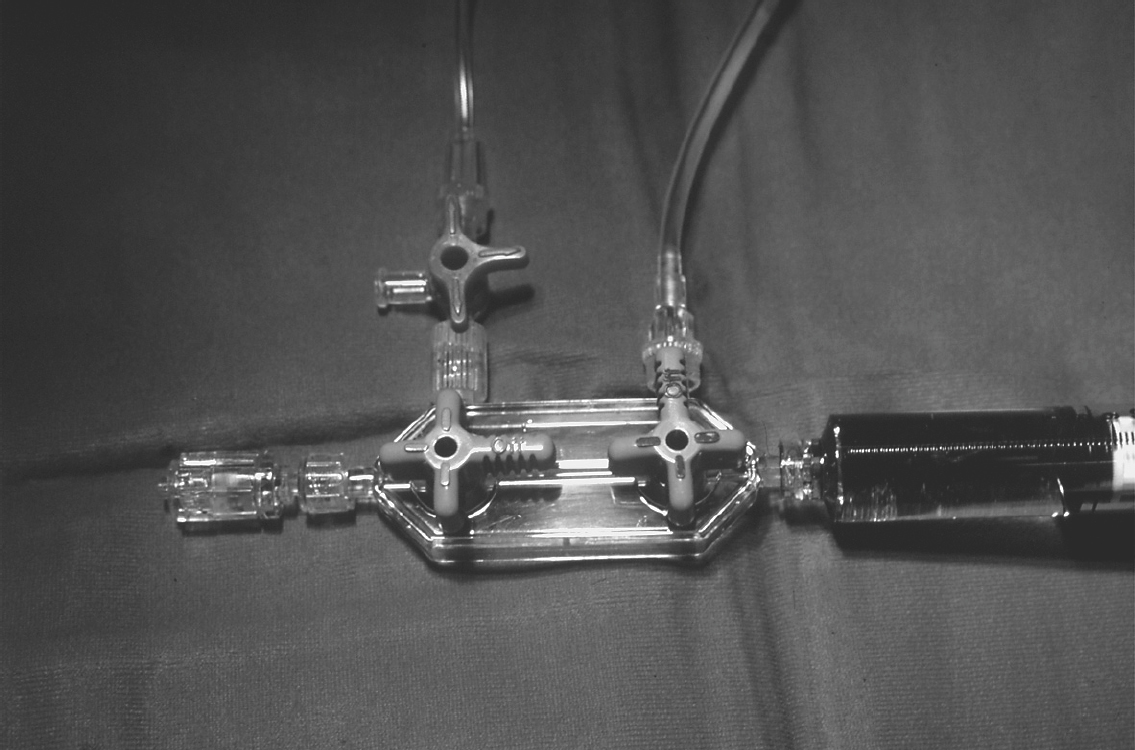
A manifold with rotator, consisting of two or three 3-way stopcocks (Fig. 8.1), in a closed system allows alternation of
 Pressure recording
Pressure recording
 Injection of contrast medium
Injection of contrast medium
 Flushing of the catheter
Flushing of the catheter
 Intra-arterial or intracoronary administration of drugs
Intra-arterial or intracoronary administration of drugs
Catheters
Catheters for coronary angiography are made of polyethylene or polyurethane and are available in different sizes. Polyurethane catheters have a relatively soft tip and low thrombogenicity. To achieve sufficient torsional stability the catheter wall frequently contains a fine wire mesh or the catheter shaft is made of nylon. The catheter length is usually 100 cm, but 125-cm catheters are also available.
Typically 4F or 5F catheters are employed. The major advantage compared with larger sizes is the shortened bed rest due to the smaller puncture site and reduced vascular complications. However, this advantage is at the expense of a smaller lumen with a corresponding limitation in contrast flow and less opacification of the coronary arteries (internal diameter with 4F is 0.042 in., with 5F is 0.045 in., with 6F is 0.056 in., and with 7F is 0.064 in.). Furthermore, thinner catheters have less torque control. It is of utmost importance to recognize in a timely fashion the technical limitations of 4F and 5F catheters if difficult anatomy is encountered.
Personal view
In the following situations we use 6F or 7F catheters either right from the start or early during the procedure, rather than manipulating for an inordinately long time with a smaller system.
 Inadequate torque control and catheter placement with
Inadequate torque control and catheter placement with
– Kinking of the iliac arteries and aorta
– Dilated thoracic aorta (hypertension, aortic disease, aortic aneurysm)
 Insufficient contrast of the coronaries with
Insufficient contrast of the coronaries with
– High flow rates in the coronary arteries (volume overloaded left ventricle with aortic and mitral regurgitation, dilated cardiomyopathy)
– Obesity
– Emphysematous thorax
A variety of preshaped catheters are available to selectively engage the coronary arteries, vein grafts, and internal mammary arteries (Figs. 8.2 and 8.3).
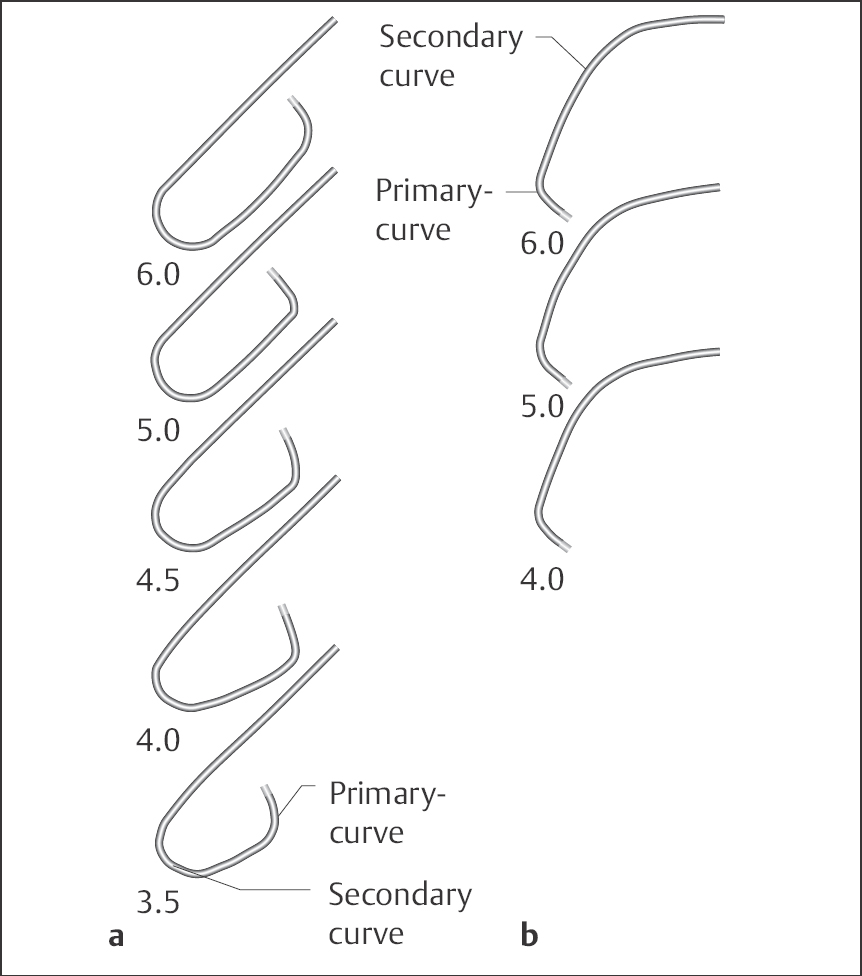
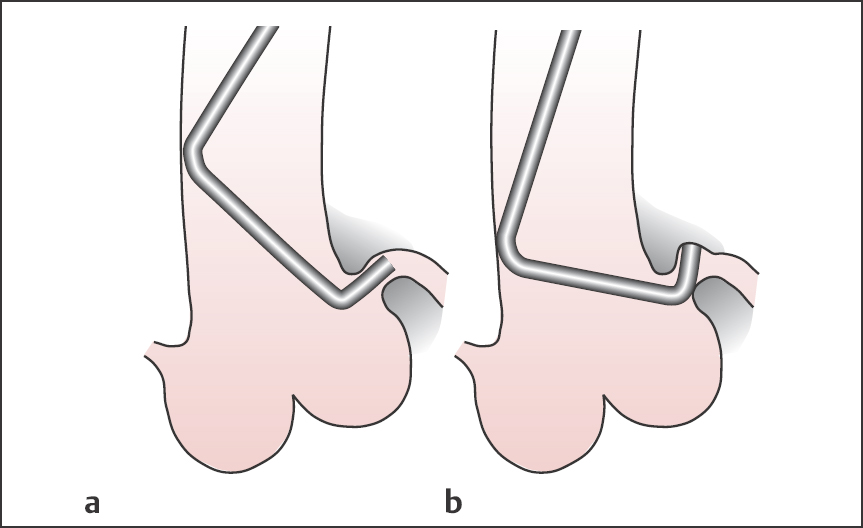
Judkins catheter (Fig. 8.2). This is the standard catheter for coronary angiography. It has an open end without side holes. Studies can be successfully performed in more than 90 % of patients. The catheters are preshaped for either the right or left coronary artery and are manufactured in different sizes, which reflect the distance between primary and secondary curves (Fig. 8.2). For a Judkins-4 catheter this distance is ~4 cm, for a Judkins-5 catheter ~5 cm, and so on. Size 4 Judkins catheters for the right and left coronaries are suitable for the average-sized adult with an ascending aorta of normal width (available sizes: 3.5, 4.0, 5.0 and 6.0). We also routinely use standard Judkins catheters for radial artery access.
Fig. 8.2 a, b Judkins coronary angiography catheter.
a Catheter for the left coronary artery, sizes 3.5 to 6.
b Catheter for the right coronary artery, sizes 4, 5 and 6.
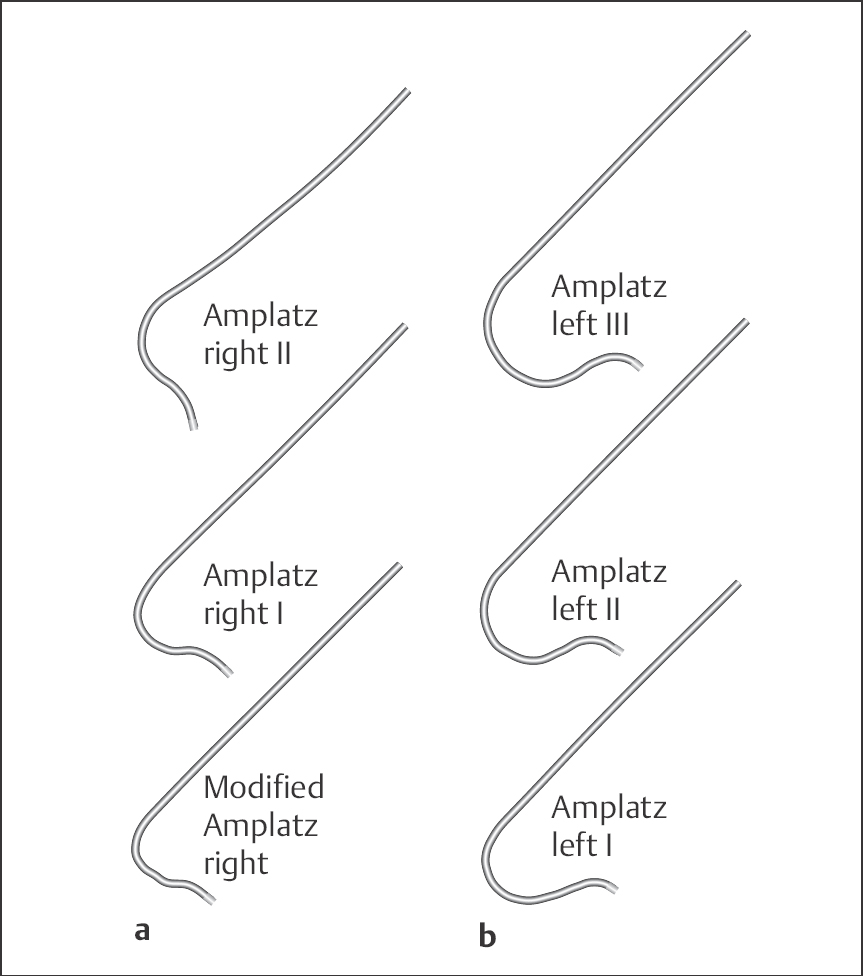
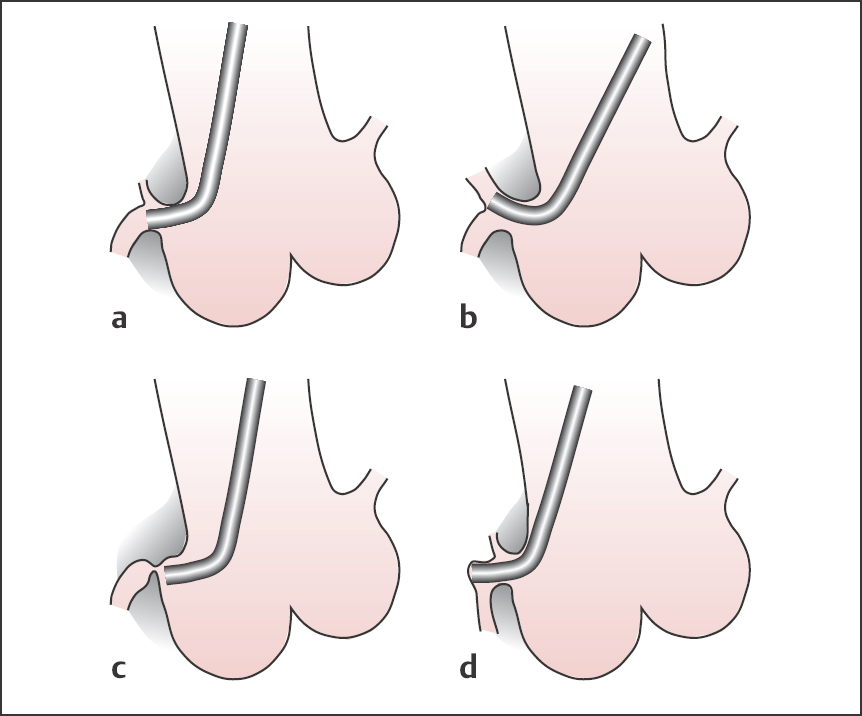
Amplatz catheter (Fig. 8.3). Amplatz catheters can be used as an alternative to the Judkins technique: these are preshaped catheters for the right or left coronary arteries with open end and no side holes. The size of the most distal curve of the left Amplatz depends upon the width of the aortic root and is available in the sizes I = small, II = normal and III = large. For engagement of the right coronary artery, a modified Amplatz catheter is used, which is available in only one size.
Coronary vein bypass graft catheter (Fig. 8.4). These are preshaped catheters for the selective engagement of vein grafts and internal mammary arteries. The right coronary vein bypass catheter can be used as an alternative to the right Judkins catheter for vein grafts to the right coronary artery; the left coronary bypass catheter can be used for vein grafts to the circumflex and left anterior descending artery (LAD) including side branches. The internal mammary artery catheter has a tighter distal curve and longer tip than the right Judkins catheter.
Additional catheters (Fig. 8.4). The Sones catheter is used for the selective angiography of both coronary arteries as well as for ventriculography and aortography. The catheter has an open end and two side holes close to the tapered catheter tip. Three sizes are available with different tip lengths: size I with 2.5-cm tip, size II with 4-cm tip, and size III with 7.6-cm tip. Multipurpose catheters are especially suited for graft imaging.
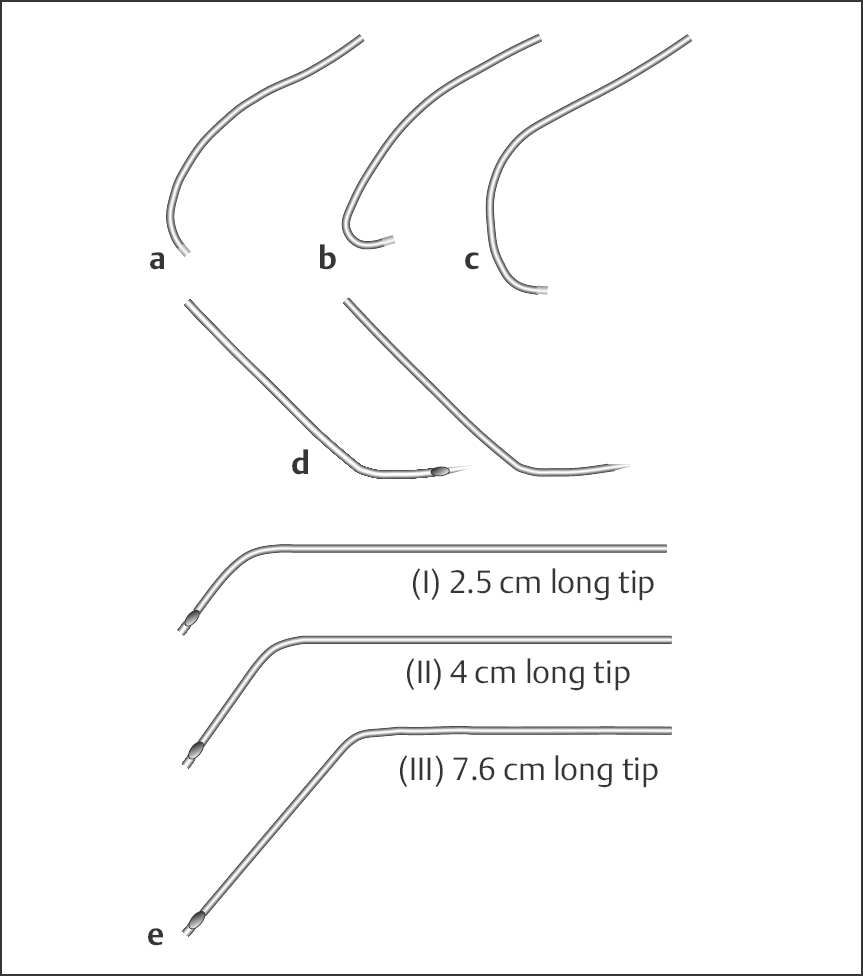
Fig. 8.4 a–e Additional catheters.
a Right coronary bypass graft catheter.
b Catheter for internal mammary artery.
c Left coronary bypass graft catheter.
d Multipurpose catheter.
e Sones catheter.
Catheterization Technique
General Rules for Cardiac Catheter Handling
 Before every examination, catheters are checked for integrity, patency of the lumen, and compatibility with the guidewire and sheath.
Before every examination, catheters are checked for integrity, patency of the lumen, and compatibility with the guidewire and sheath.
 The catheter is advanced carefully together with the guidewire, which protrudes ~5 to 10 cm from the catheter tip, to the descending thoracic aorta or with the wire to the ascending aorta.
The catheter is advanced carefully together with the guidewire, which protrudes ~5 to 10 cm from the catheter tip, to the descending thoracic aorta or with the wire to the ascending aorta.
 Every manipulation of the catheter or guidewire should be done under fluoroscopy.
Every manipulation of the catheter or guidewire should be done under fluoroscopy.
 Every manipulation has to be done gently and without force.
Every manipulation has to be done gently and without force.
 After removal of the guidewire, blood is aspirated from the catheter to remove air and smaller thrombi. The catheter is then flushed with saline. This is done after every catheter exchange and after every disconnection from the manifold. Usually arterial blood flows back from the catheter spontaneously and no force is required for aspiration.
After removal of the guidewire, blood is aspirated from the catheter to remove air and smaller thrombi. The catheter is then flushed with saline. This is done after every catheter exchange and after every disconnection from the manifold. Usually arterial blood flows back from the catheter spontaneously and no force is required for aspiration.
 If blood cannot be aspirated, then most likely the catheter tip is impinging on the aortic wall. If no blood aspiration is possible even after correcting the catheter position, reopening the catheter by injecting saline or reintroducing the guidewire should never be attempted. The catheter has to be removed.
If blood cannot be aspirated, then most likely the catheter tip is impinging on the aortic wall. If no blood aspiration is possible even after correcting the catheter position, reopening the catheter by injecting saline or reintroducing the guidewire should never be attempted. The catheter has to be removed.
 After aspiration of blood and flushing, the catheter is connected with the flush solution running to the manifold. From this moment on, arterial pressure is continuously recorded until the catheter is removed.
After aspiration of blood and flushing, the catheter is connected with the flush solution running to the manifold. From this moment on, arterial pressure is continuously recorded until the catheter is removed.
 With every manipulation the torque control of the catheter has to be considered; that is, every rotation of the catheter by the operator has to correspond to a rotation of the catheter in the aorta. If that is not the case, continuation of the manipulation can lead to kinking of or formation of a knot in the catheter shaft. In most cases the cause is kinking of the iliac arteries and may require the use of a stiffer catheter or even a different access route. Alternatively, engagement of the coronary ostium can be achieved with the guidewire (0.035 in.) in place. Here the wire still has to be safely inside the catheter (~5 cm proximal to the tip). The end of the wire is being led to the outside via a Y-connector. If the catheter lumen is of sufficient size (≥ 6F), the catheter can be rotated safely with the possibility of intermittent opacification.
With every manipulation the torque control of the catheter has to be considered; that is, every rotation of the catheter by the operator has to correspond to a rotation of the catheter in the aorta. If that is not the case, continuation of the manipulation can lead to kinking of or formation of a knot in the catheter shaft. In most cases the cause is kinking of the iliac arteries and may require the use of a stiffer catheter or even a different access route. Alternatively, engagement of the coronary ostium can be achieved with the guidewire (0.035 in.) in place. Here the wire still has to be safely inside the catheter (~5 cm proximal to the tip). The end of the wire is being led to the outside via a Y-connector. If the catheter lumen is of sufficient size (≥ 6F), the catheter can be rotated safely with the possibility of intermittent opacification.
 Prior to injection of contrast into the coronary arteries a test injection of a small volume of contrast is done in the aorta.
Prior to injection of contrast into the coronary arteries a test injection of a small volume of contrast is done in the aorta.
 For engagement of the coronary arteries the catheter has to be stably seated in the respective ostium without tension. Further manipulation of the catheter during or between contrast injections should be avoided.
For engagement of the coronary arteries the catheter has to be stably seated in the respective ostium without tension. Further manipulation of the catheter during or between contrast injections should be avoided.
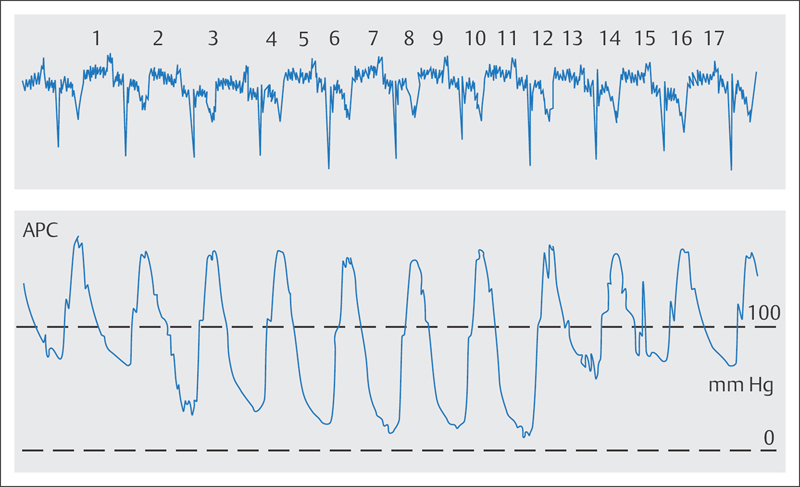
 The pressure tracing and the ECG have to be carefully monitored during every manipulation while engaging the coronary arteries. If engagement of the coronary arteries results in a pressure drop or in a so-called ventricularization of the pressure tracing (Fig. 8.5), then the catheter has to be pulled back into the aorta immediately. The catheter should be seated safely in the ostium so that the contrast is injected completely into the coronary artery. This provides good opacification and reduces contrast exposure at the same time.
The pressure tracing and the ECG have to be carefully monitored during every manipulation while engaging the coronary arteries. If engagement of the coronary arteries results in a pressure drop or in a so-called ventricularization of the pressure tracing (Fig. 8.5), then the catheter has to be pulled back into the aorta immediately. The catheter should be seated safely in the ostium so that the contrast is injected completely into the coronary artery. This provides good opacification and reduces contrast exposure at the same time.
 When injecting into the coronary arteries, the syringe content must never be injected completely due to the risk of air embolism. For the same reason, the tip of the syringe should always be pointed down.
When injecting into the coronary arteries, the syringe content must never be injected completely due to the risk of air embolism. For the same reason, the tip of the syringe should always be pointed down.
 Catheter exchange always has to be done over a guidewire. However, due to the risk of thrombus formation, guidewires may remain only briefly in the vasculature.
Catheter exchange always has to be done over a guidewire. However, due to the risk of thrombus formation, guidewires may remain only briefly in the vasculature.
 New operators should familiarize themselves with the geometric and technical properties of a catheter before its introduction. They will thus know prior to catheterization whether a clockwise or counterclockwise rotation of the catheter will lead to an anterior or posterior rotation of the catheter tip.
New operators should familiarize themselves with the geometric and technical properties of a catheter before its introduction. They will thus know prior to catheterization whether a clockwise or counterclockwise rotation of the catheter will lead to an anterior or posterior rotation of the catheter tip.
 Judkins Technique
Judkins Technique
The Judkins technique (Fig. 8.6) is currently the standard technique for engaging the left and right coronary artery.
Engagement of the Left Coronary Artery
The left coronary artery originates ~1 cm above the aortic valve from the left posterolateral aorta. The left Judkins catheter is advanced together with the guidewire, which juts out from the catheter, up to the descending aorta or, alternatively, around the aortic arch up into the ascending aorta. The wire is removed, blood is carefully aspirated, and the catheter is flushed and subsequently connected to the manifold.
Engagement of the left coronary artery is usually done in the 40° left anterior oblique (LAO) projection, in which the origin of the artery is projected on the left side of the aorta. This projection allows for the precise evaluation of the catheter position in relation to the ostium of the left coronary artery. Furthermore, the fit of the selected Judkins catheter size in relation to the now recognizable width and course of the ascending aorta can be assessed.
Alternatively, the left coronary artery can be engaged in the anteroposterior (AP) projection (0°) or in right anterior oblique (RAO) 30° projection. In particular stenoses of the left main stem can be recognized quickly and easily in this projection, and therefore some operators prefer it.
Engagement of the left coronary artery with the Judkins technique is relatively easy: Usually the catheter only needs to be advanced slowly under fluoroscopy without any further manipulation, and the catheter tip enters the left coronary ostium. After checking the pressure tracing and ECG, a small amount of contrast medium is injected to confirm the correct catheter position and to get a general assessment of the left coronary main stem. The catheter then remains without further manipulation in the ostium until completion of the angiograms.
Troubleshooting
Damping or ventricularization of the pressure wave (Fig. 8.5). The catheter must always be pulled out of the ostium without delay when damping or ventricularization occurs. Not infrequently, the cause is left main stem stenosis, which has to be confirmed or excluded before the examination is continued. In most cases a good evaluation of the main stem can be achieved by moving the catheter into close proximity of the ostium without entering it again, and then injecting contrast. After that it can be decided whether a careful new engagement is advisable. With distal left main stem stenosis, the catheter tip can be kept at an appropriately safe distance from the stenosis, and therefore contrast can be carefully injected if the pressure curve is normal. In general both duration of the catheter dwelling in the ostium and the number of contrast medium injections should be minimized with left main stenosis.
In addition, incorrect positioning of the catheter, in which the tip impinges on the coronary artery wall, can cause a damping of the pressure curve; this is frequently seen if the selected Judkins curve for the left coronary artery is too small (Fig. 8.7). Contrast injection should be avoided with this catheter position due to the risk of dissection. Occasionally this catheter position can be corrected by a slight counterclockwise turn. If this fails, the catheter has to be exchanged and the next larger curve size should be selected. If in doubt, also replace it in the case of a normal pressure curve and a steep position of the catheter tip (distal catheter end lies against the upper arterial wall), as the recoil during contrast medium injection not infrequently leads to disengagement of the catheter. This would result in a correspondingly weak opacification of the coronary artery; furthermore, there is always the risk of catheter-induced dissection of the left main stem.
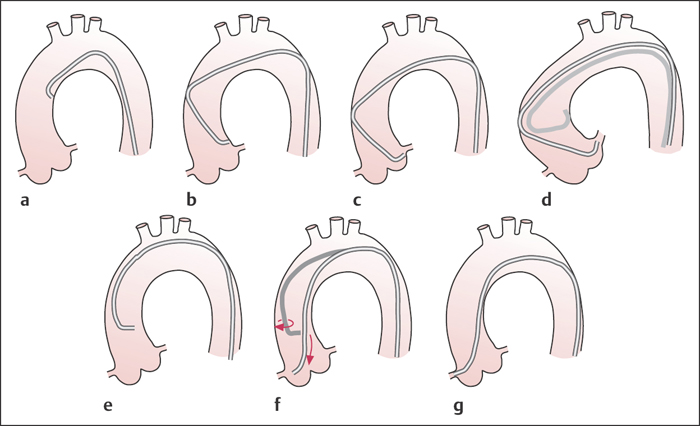
Fig. 8.6 a–g Judkins technique to engage the left and right coronary artery (LAO projection).
a–c To engage the left coronary ostium with the catheter, it is usually sufficient to slowly advance the catheter under fluoroscopy without further manipulation. The catheter tip essentially finds the left coronary ostium by itself.
d If the ascending aorta is widened, a larger Judkins curve is required; the 4 curve is usually too small and when advanced the catheter kinks.
e–g To engage the right coronary artery, either the right Judkins catheter is positioned 2 to 4 cm above the aortic valve and without retraction turned clockwise, or it is advanced to the aortic valve and then turned clockwise with slight retraction.
Fig. 8.7 a, b Engagement of the left coronary artery with the Judkins technique.
a Correct catheter position.
b Incorrect catheter position.
High origin of the left coronary artery. If the origin of the left coronary artery is located high and posteriorly, sometimes it is possible to engage the ostium simply by turning the Judkins catheter counterclockwise. However, more frequently this results in kinking of the catheter, so that here the use of a left Amplatz catheter is more likely to be successful (for technique see p. 45).
Short left coronary main stem or separate ostia for the LCX and the LAD. If the common main stem is only very short or missing completely, the standard Judkins catheter often only allows the superselective engagement either of the left circumflex artery (LCX) or of the LAD; the other branch can be visualized only inadequately, if at all. If the Judkins-4 catheter initially enters the LAD when advanced in the usual manner, it may be possible to enter the LCX by slightly pulling back the catheter with clockwise rotation. It this is not successful a larger Judkins curve or a left Amplatz catheter should be used.
Conversely, with initial selective engagement of the LCX with the standard Judkins catheter, careful counterclockwise rotation can be attempted to enter the LAD. However, in many cases this is only successful after changing to the next smaller Judkins size.
Dilated aortic root/kinking of the catheter. If the aortic root is wide, successful imaging of the left coronary artery is rare with the Judkins-4 catheter; catheter manipulation usually results in kinking of the catheter in the ascending aorta. Engagement is often possible after exchanging the catheter for a Judkins with a larger curve. A left Amplatz catheter can be tried if necessary.
Engagement of the Right Coronary Artery
Catheterization of the right coronary artery is done using a 60° or 40° LAO projection. The preshaped Judkins right catheter is advanced to the level of the aortic root. In this position the catheter tip almost always points left. Under fluoroscopy and with constant monitoring of the pressure curve and the ECG, the catheter is rotated clockwise by 90° to 180° and at the same time slowly pulled back a little (2–3 cm). This makes the catheter tip turn right toward the right coronary ostium, which is located anteriorly; the rotation also lowers the tip, which is compensated for by pulling back. Alternatively, the Judkins right catheter can be placed 2 to 4 cm above the aortic valve and rotated clockwise without pulling back.
Successful engagement of the right coronary ostium is frequently indicated by a sudden movement of the catheter tip to the right. After checking the pressure curve and the ECG, the catheter position can be verified with a careful test injection of contrast.
Troubleshooting
Damping/ventricularization of the pressure curve. In this situation, the first action to take should always be to pull back the catheter. A pressure drop is much more common with engagement of the right coronary artery (RCA) than with the left coronary artery. There are multiple causes for pressure damping with RCA engagement (Fig. 8.8):
 Higher-grade ostial stenosis
Higher-grade ostial stenosis
 Catheter-induced coronary spasm
Catheter-induced coronary spasm
 Subselective engagement of the conus branch of the RCA
Subselective engagement of the conus branch of the RCA
 Small RCA
Small RCA
 Complete occlusion close to the ostium
Complete occlusion close to the ostium
 Impingement of the catheter tip on the arterial wall
Impingement of the catheter tip on the arterial wall
The exact cause for pressure damping in an individual case can only be determined by angiography. In some cases semiselective imaging of the proximal right coronary artery can be achieved by forceful injection of contrast into the right coronary sinus. Usually the right coronary artery has to be engaged again, but the catheter has to be pulled back immediately after careful contrast medium injection to image the cause of the pressure damping. Subsequently it can be decided which measures are required to continue the study:
Fig. 8.8 a–d Engagement of the right coronary artery with the Judkins technique.
a Correct catheter position.
b Pressure damping due to selective engagement of the conus branch.
c Pressure damping due to severe ostial stenosis.
d Pressure damping due to catheter tip impinging on the wall.
 A severe ostial stenosis requires careful catheter positioning and contrast medium injection due to the risk of dissection.
A severe ostial stenosis requires careful catheter positioning and contrast medium injection due to the risk of dissection.
 With complete occlusion of the RCA further contrast medium injection is unproblematic.
With complete occlusion of the RCA further contrast medium injection is unproblematic.
 If the artery is small the injection should be performed with caution so as to avoid arrhythmias.
If the artery is small the injection should be performed with caution so as to avoid arrhythmias.
 A subselective catheter position with engagement of a conus branch has to be corrected before further contrast medium is injected as this regularly induces ventricular fibrillation. Frequently the placement can be corrected with slight rotation or pulling back of the catheter. If necessary a modified right Amplatz catheter may provide a good position.
A subselective catheter position with engagement of a conus branch has to be corrected before further contrast medium is injected as this regularly induces ventricular fibrillation. Frequently the placement can be corrected with slight rotation or pulling back of the catheter. If necessary a modified right Amplatz catheter may provide a good position.
 If catheter-induced coronary spasm is suspected, sublingual or intracoronary nitroglycerin is given before repeating imaging of the right coronary artery. In addition, the catheter position should be checked and corrected if necessary (e.g., if the catheter tip is not aligned with the vessel but tips upward or downward, or engagement is too deep).
If catheter-induced coronary spasm is suspected, sublingual or intracoronary nitroglycerin is given before repeating imaging of the right coronary artery. In addition, the catheter position should be checked and corrected if necessary (e.g., if the catheter tip is not aligned with the vessel but tips upward or downward, or engagement is too deep).
Disengagement of the catheter. During angiography the right Judkins catheter should be seated stably and without tension in the ostium of the right coronary artery. Not infrequently, however, the catheter springs out of the ostium again. The cause of this is usually too much rotation of the catheter during the initial engagement, with the stored rotational energy ultimately leading to spontaneous rotation of the catheter. As a result either the catheter is disengaged or it enters the coronary artery too deeply. Slight counterclockwise rotation of the catheter reduces or eliminates this stored rotational energy and a stable seating is achieved.
 It is important to consider adequate torsion control of the catheter even for the first engagement attempt.
It is important to consider adequate torsion control of the catheter even for the first engagement attempt.
Difficult RCA engagement. The right coronary artery has more variations in the location of the ostium than the left coronary artery, without these necessarily being true anomalies. Therefore, engagement with the standard Judkins technique is frequently difficult or impossible. With a forceful contrast medium injection into the right coronary sinus, the location of the ostium can usually be identified, facilitating catheter placement:
 If the ostium is located only slightly anteriorly, lateral fluoroscopy (90°) can facilitate engagement with the Judkins technique.
If the ostium is located only slightly anteriorly, lateral fluoroscopy (90°) can facilitate engagement with the Judkins technique.
 With anterior location or origin at a higher level (above the sinotubular junction), right ostial engagement is frequently very successful with a left Amplatz catheter.
With anterior location or origin at a higher level (above the sinotubular junction), right ostial engagement is frequently very successful with a left Amplatz catheter.
The left Amplatz catheter is also suitable for dilated aortic roots. Alternatively, a multipurpose catheter (Fig. 8.4) can be used.
 Amplatz Technique
Amplatz Technique
The Amplatz technique is illustrated in Fig. 8.9.
Engagement of the Left Coronary Artery
In the following situations the Amplatz technique can be a helpful or necessary alternative to the Judkins technique for engaging the left coronary artery:
 Dilated aortic root (aortic regurgitation and stenosis, hypertension, etc.)
Dilated aortic root (aortic regurgitation and stenosis, hypertension, etc.)
 High origin of the left coronary artery
High origin of the left coronary artery
 Separate ostia for LCX and LAD
Separate ostia for LCX and LAD
 Short main stem with subselective engagement of LAD
Short main stem with subselective engagement of LAD
Engagement is done using the 40° LAO or 30° RAO projection. For a normal—sized adult, the standard size II is usually suitable.
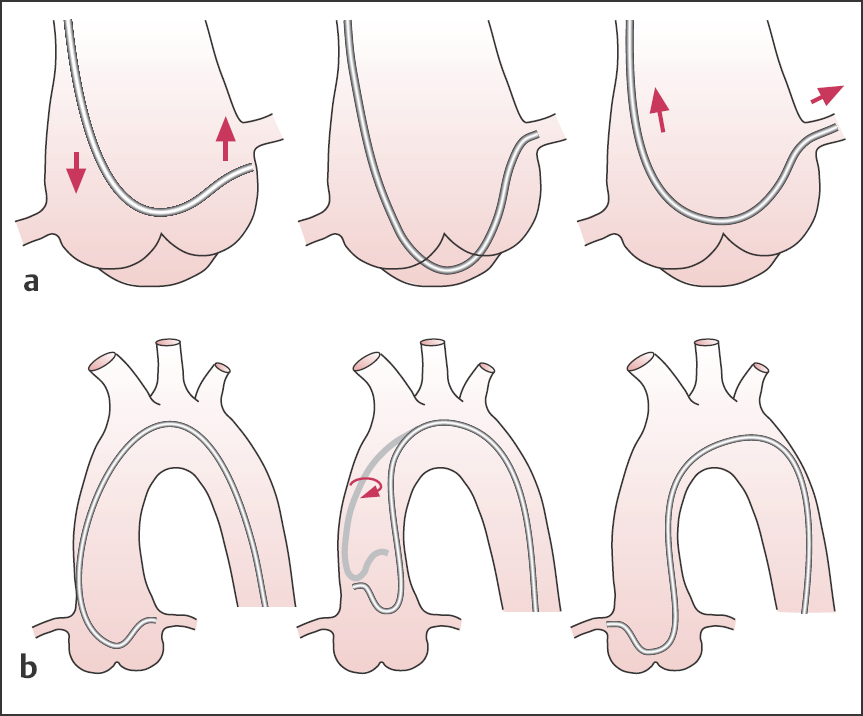
The pressure curve and ECG are constantly monitored while the catheter with the tip pointing left and caudally is advanced around the aortic arch to the left sinus of Valsalva. Further advancement of the catheter moves the catheter tip cranially with engagement of the left coronary ostium (Fig. 8.9). Engagement occurs not infrequently with a sudden jerk. If the catheter is further advanced, the catheter tip may disengage from the ostium. In contrast, a slight pulling back of the Amplatz catheter after placement in the left coronary ostium can result in deeper engagement, potentially with selective catheterization of the LCX.
If the curve of the Amplatz catheter is too small, the catheter tip cannot reach the ostium; if the curve is too big, the catheter cannot be properly guided to the level of the aortic root and the ascending aorta.
Fig. 8.9 a, b Amplatz technique.
a Engagement of the left coronary artery.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree