MISSED LUNG CANCER
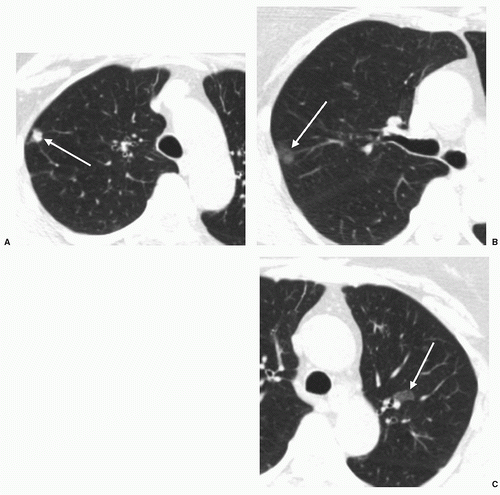
Experience teaches that larger lesions are more easily diagnosed than smaller lesions, and peripheral lesions are more readily detected than central lesions. Radiologic diagnosis is facilitated by the presence of typical radiographic features; uncommon manifestations of lung cancer, such as spontaneous regression, may prove misleading.
6,
7 In one study of 27 missed lung cancers, the single most frequently identified cause of missed diagnoses was failure of the radiologist to compare the current chest radiographs with previous chest radiographs.
8 Other important factors were upper lobe location of the lesion (81%) and female gender of the patient (67%). A follow-up study from the same institution
9 featured 40 missed non-small cell lung cancers (NSCLC) over an 8-year period. In this series, gender did not play a role, but 72% of missed lesions were again in the upper lobes, and 22% were obscured by a clavicle.
Application of computerized automated lung nodule detection methods (computer-aided diagnosis [CAD]) to digital chest radiographs may improve the detection rate of lung cancers. One preliminary study using a commercial, computerized detection system on radiographs with T1 lesions showed a detectability rate of 74% (37/50) using CAD.
10 However, the CAD system showed a false-positive rate of 2.3 per case in 50 normal chest radiographs. The mean area under the receiver operating characteristic (ROC) curve for all observers increased significantly from 0.896 without CAD to 0.923 with CAD in the lung cancer cases. The primary reason for the improvement in performance was caused by a decrease in the number
of false negatives and a concomitant increase in the number of true positives.
10The novel technique of single-exposure, dual-energy digital chest radiography appears promising for the detection of pulmonary nodules and lung cancers. In a preliminary study assessing the detectability of lung nodules in 77 patients with lung cancer and 77 normal subjects, the combination of standard radiography and single-exposure, dual-energy digital radiography improved nodule detection compared to standard radiography alone.
11Some authors have analyzed the detectability of lung cancers based on size and extent of ground-glass opacity at thinsection CT.
12 They reviewed 75 peripheral NSCLC (26 localized BACs and 49 other cell types). The chest radiographs were mixed with 60 normals and blindly reviewed. Sensitivity for detection was 58.5% for BACs and 78.6% for the other cell types. Lesions <15 mm in size and those with ≥70% groundglass opacity proved to be statistically significantly more difficult to detect. A similar result was obtained in a study analyzing lung cancers missed at low-dose helical CT screening.
13 In this study, 32 of 83 lung cancers found by annual low-dose CT s creening had initially been missed. Nodules missed because of detection errors more frequently were ground glass (91%), and also, more frequently were judged to be “subtle” (91%).
Application of a computerized automated lung nodule detection method to proven lung cancers missed at low-dose CT screening may improve the detection rate significantly.
14 In a series of 38 missed lung cancers, such a method allowed identification of 32 (84%) of the missed lesions.
Similar to its use in chest radiography, CAD is an emerging tool for automatic detection of pulmonary nodules on CT; it may be used as a second reader, drawing the attention of the radiologist to possible abnormalities in order to increase the detection rate of small pulmonary nodules. High-resolution data (thin sections obtained during a single breath hold) acquired with multidetector CT (MDCT) has led to improvements in the sensitivity of various CAD systems and a decrease the false-positive rate. There are diverse methods of CAD, each based on different detection algorithms.
15 One recent study compared two CAD systems in 25 patients with 116 nodules, evaluated by three radiologists with varying levels of expertise
16; this study showed that sensitivity for pulmonary nodule detection increased with the use of CAD. For example, in nodules smaller than 5 mm, sensitivity increased from 55% to 71% to 68% to 74%. There was no significant difference in the performance of the two CAD systems.
Other CT evaluation methods have also been shown to decrease perception error and improve detection of pulmonary nodules. These dedicated computer applications involve alternative two- (2D) and three-dimensional (3D) displays of CT data.
17,
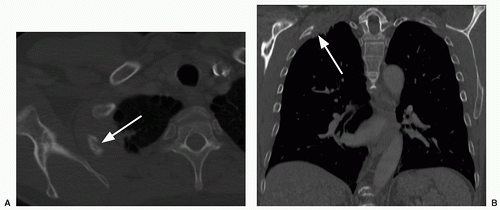
18 For example, on maximum intensity projection (MIP) images, pulmonary nodules tend to stand out against background structures, such as lung and tubular vessels (
Fig. 26.6); several studies have demonstrated the superiority of MIP over standard axial images. In one study, five readers evaluated examinations of 25 patients with 122 nodules (3 to 9 mm in diameter). Readings were performed with and without MIP.
17 MIP enhanced the detection of peripheral nodules for the junior readers, and of central nodules for both the junior and senior readers. Volume rendering (VR) in 3D techniques display the entire volume data, assigning relative opacity values to each voxel (ranging from 0% to 100%). In a study comparing MIP and VR computer applications among three readers, VR was superior to MIP in the detection of pulmonary nodules smaller than 11 mm and was associated with a statistically significant shorter reading time.
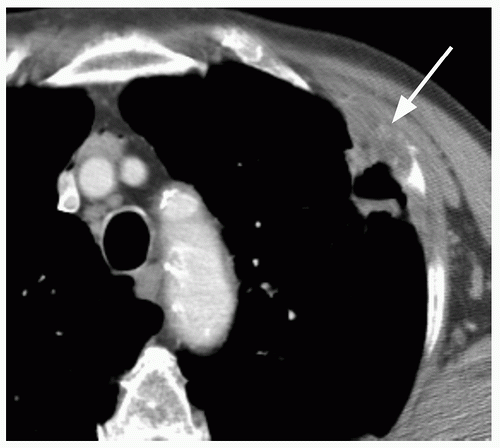
18Is missed lung cancer automatically evidence of malpractice? In the Mayo Clinic lung cancer screening article
previously cited,
19 each radiographic study was reviewed by two (and often three), trained and interested observers (chest radiologists or chest physicians) specifically to answer the question: “Is there lung cancer?” Amazingly, 45 of 50 peripheral carcinomas that they diagnosed were visible in retrospect, with 18 visible for more than 1 year and four for more than 2 years; one was visible in retrospect for 53 months. Furthermore, 12 of 16 perihilar carcinomas and 13 of 20 carcinomas presenting as hilar or paratracheal lymph node enlargement were visible in retrospect, although not generally for as long as the peripheral carcinomas. The authors concluded that “… failure to detect a small pulmonary nodule on a single examination should not constitute negligence or be the basis for malpractice litigation.” In an elegant letter to the editor, Hendrix
20 pointed out the need for an analogy to explain to members of a lay audience (the jury) how a well-qualified, careful radiologist could miss the lesion that they now easily see on a radiograph. He likens the radiologist’s analysis of the image to the search for Waldo in the series of
Where’s Waldo? books. As Dr. Hendrix pointed out, everyone understands how hard it can be to locate Waldo in a given illustration. However, once he has been found, Waldo is amazingly obvious when the same illustration is reviewed. As Dr. Hendrix added, it is even harder to look for lung cancer (or any other radiographic finding) because, although Waldo is definitely present on every page of a
Where’s Waldo book, a radiograph may be normal. Even with these potentially helpful legal strategies, defending missed lung cancer is generally an unpleasant experience.
21
SOLITARY PULMONARY NODULE
The SPN is a common presentation of lung cancer. However, most SPNs are benign. Summarizing five large series of resected SPNs seen on chest radiographs,
22,
23,
24,
25,
26 Siegelman et al.
27 noted that 53.9% were granulomas, 28.3% were bronchogenic carcinomas or other primary malignancies, 6.6% were hamartomas, and 3.5% were metastases. An even higher percentage of all radiographically detected SPNs are presumably benign, because nodules that appear calcified on chest radiographs are rarely resected.
The challenge in evaluating SPNs is to avoid invasive procedures in patients who have benign nodules without allowing potentially curable bronchogenic carcinomas the time to progress to more advanced or even unresectable disease. This is an area of active, ongoing research; however, the many approaches that have been tried attest to the lack of complete success for most modalities to date. A proper SPN evaluation acknowledges the following key points:
Imaging at a single point in time relies heavily on morphologic characteristics in distinguishing benign from malignant SPNs.
Calcification is the single best morphologic indicator of benignancy.
Behavior (i.e., lack of growth) is far better than any morphologic criterion at predicting benignancy.
Any predictor of benignancy must err on the side of intervention—it is better to resect a benign SPN unnecessarily than erroneously to call a malignant SPN benign.
With these key points in mind and realizing the significant expense (and in some cases radiation dose) of radiologic tests, it is always best to start the evaluation of the SPN by seeking old radiographs for comparison. This saves money, radiation, and often time, and provides the possibility for proving that a lesion is benign, no matter what its morphology is. A lesion that is stable for 2 years or more is considered to be benign, although the exception occurs for ground glass nodules at CT, which may represent very indolent adenocarcinomas. The flip side is that almost no matter what the morphology is, a growing lesion has declared itself to be one that should be resected. The lack of vigor with which old films are pursued is generally disappointing; if the patient were a close relative, we would all try a lot harder to spare him or her unnecessary tests that involve (potentially fatal) injection of intravenous contrast. And consider this—how many adults 40 years of age or older have never had a prior chest radiograph? In the United States, the number must be vanishingly small.
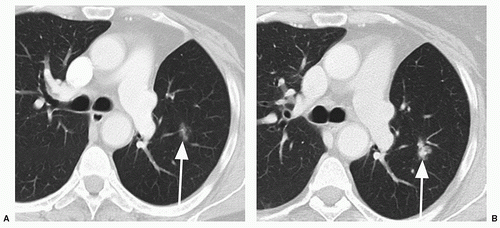
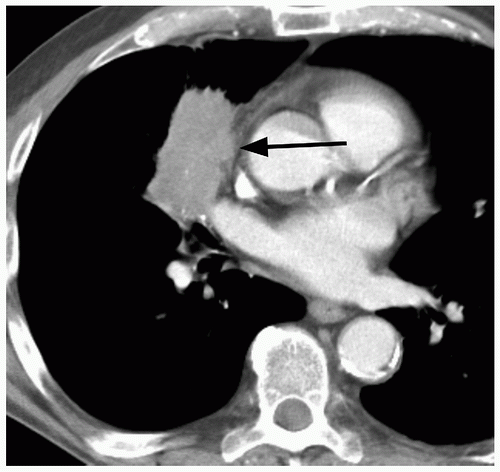
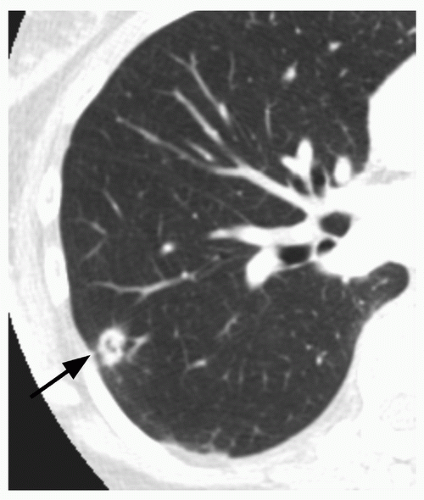
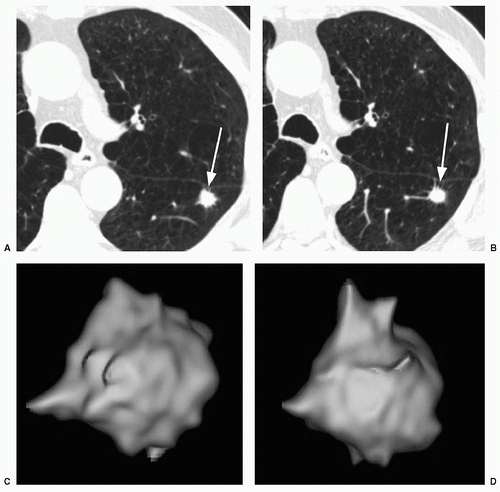
Whereas the concept of stability appears, on the surface, to be fairly straightforward, in practice it can be quite difficult to determine if a nodule has grown, particularly if it is small (e.g., less than 1 cm in diameter). This is true for both conventional radiography and for CT. For instance, a nodule that has increased from 10 to 11 mm in diameter may show no apparent, significant change in size at radiography or on axial CT scans; however, this represents a volume increase of 33%. To maximize the ability to detect such changes in size, it is important to optimize both CT imaging parameters, as well as postprocessing techniques. Particularly for small nodules, the best results may be obtained using thin section (1 to 2.5 mm), overlapping CT sections with 3D volumetric reconstructions (
Fig. 26.7).
28,
29 In addition, all follow-up scans should be performed using the same techniques. Volumetric measurements may be affected by many factors, including section thickness and spacing, x-ray dose, motion artifact, respiratory or cardiac phase, nodule location, and intraobserver/extraobserver variability; therefore, in general, volume differences less than about 25% should be regarded with skepticism.
28,
30,
31,
32,
33,
34,
35,
36Sometimes, the clinical decision is made to prospectively follow an SPN with imaging, to demonstrate stability; this raises questions about appropriate scanning intervals. One study based on phantom exams and in vivo nodules, using automatic segmentation for lesion boundary definition, found that CT follow-up at 30 days could detect interval growth for all malignant lesions larger than 1 cm, and for lesions as small as 5 mm with a doubling time faster than 150 days.
37 Even for 5-mm lesions with slower doubling times, a second follow-up CT 30 days later rendered growth detectable in all cases. In a subsequent study of 13 patients, all five malignant nodules had doubling times less than 177 days, and all eight benign nodules had doubling times greater than 395 days.
38 However, other authors have found that a significant proportion of
malignant tumors have much longer doubling times (>465 days), and therefore short-term follow-up may not be helpful in many patients.
5,
29,
39,
40For a small indeterminate SPN seen at CT, follow-up guidelines have been published by the Fleischner Society
41 (
Table 26.1), as well as others,
42 regarding the suggested time intervals for repeat CT scanning, taking into account nodule size and whether the patient is clinically at low or high risk for developing lung cancer. These guidelines suggest follow-up out to 2 years, unless the nodule is nonsolid (ground glass) or partly solid; it is probably prudent to follow these nodules out to at least 3 years, because they could represent indolent adenocarcinomas, including BACs.
If a nodule is stable over a substantial period of time (e.g., 2 to 3 years), then it is almost certainly benign. However, it is well-known that not all growing nodules are malignant; various types of benign nodules may increase in size over time, including hamartomas, granulomas, and various other infectious or inflammatory lesions. In general, benign nodules tend to grow either extremely fast or extremely slowly, compared to
malignancies. Therefore, it has been postulated that growth rates of nodules may be helpful in distinguishing benign from malignant nodules. However, a recent study addressing this issue, using data based on serial, thin section CT scans, found extensive overlap among the growth rates of benign and malignant, clinically suspicious, pathologically proven lung n odules.
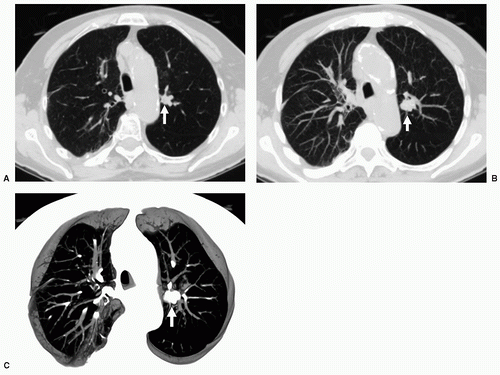
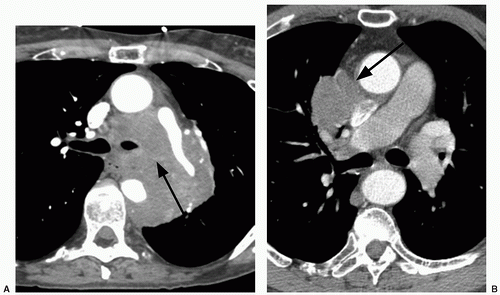
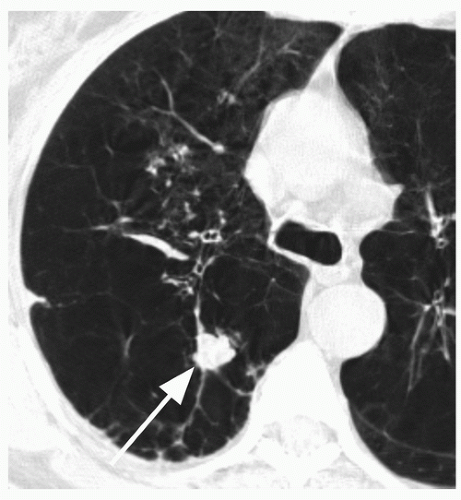
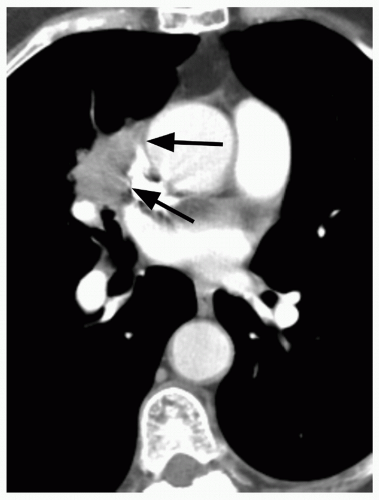
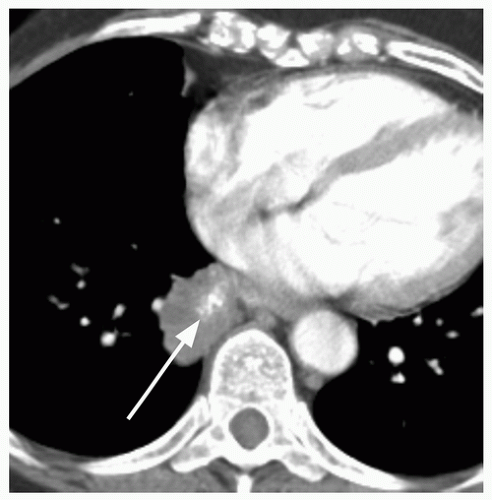
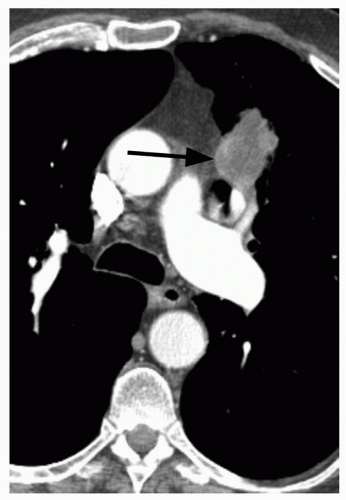
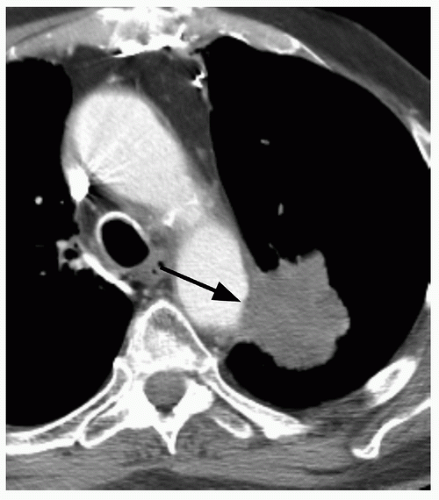
29When comparison studies are not available to establish stability, assessment of morphologic features is the next step in an SPN evaluation. Various morphologic features are suggestive of malignancy, including spiculation; ill-defined, lobulated, or irregular margins, with distortion of adjacent vessels; heterogeneity; central cavity with thick, irregular walls; and air bronchograms (
Figs. 26.1 and
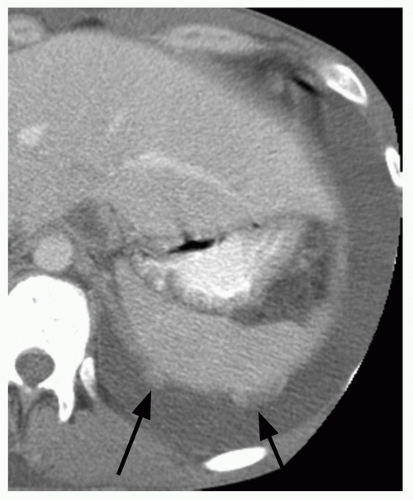
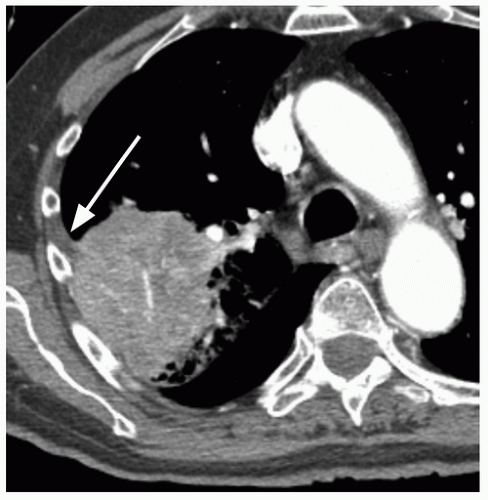
26.7). In addition, a groundglass nodular opacity on CT, particularly with a new solid component (
Fig. 26.8), adjacent pleural thickening or retraction (
Fig. 26.2), and large lesion size are features associated with malignancy. On the other hand, benign features include calcification, smooth, well-defined margins; concave, linear, branching or polygonal shape; subpleural location; homogeneous and solid opacity; and a cavity with thin, smooth walls. Unfortunately, there is great overlap in these features between benign and malignant lesions.
Helical CT was used in one study to evaluate the surface morphology of lesions, with particular attention to vascular involvement; 29 patients with noncalcified SPNs less than 3 cm in size were examined.
43 Eighteen SPNs were malignant (nine bronchogenic carcinoma, nine metastatic) and eleven were benign (six granulomas, two hamartomas, one mixed granuloma, one arteriovenous malformation, one inflammatory infiltrate). Venous involvement was present in all 18 malignant SPNs, but also in 4 of 11 benign SPNs. Arterial involvement was seen in all nine bronchogenic carcinomas, in five of nine metastatic lesions, and in 2 of 11 benign SPNs. Thus vascular involvement does not distinguish between benign and malignant SPNs.
Of all the morphologic features that can be examined, it appears that demonstration of calcification is the best way to attempt to establish a benign etiology. Unfortunately, analysis of lung nodule calcification on chest radiographs is inaccurate. In a review of 35 nodules seen on posteroanterior (PA) and lateral chest radiographs and thin-section CT, radiologists were asked to predict lesion calcification at radiography with one of six levels of confidence.
44 Among the nodules thought to be “definitely calcified,” 7% were not actually calcified. The authors suggested that nodules without documentation of long-term stability may warrant a low threshold for CT correlation. In fact, the demonstration of calcification on chest radiographs has been made more difficult by current widely used techniques that employ high kilovolt (peak). For that reason, chest fluoroscopy with low kilovolt (peak) spot films would be a good next step in looking for calcification. Unfortunately, few centers still perform chest fluoroscopy. Computed chest radiography with selective windowing may be an alternative possibility.
45 In addition, dual energy subtraction radiography is occasionally performed to improve confidence in the detection or exclusion of nodule calcification.
46At CT, assessment for calcification is currently performed by visual assessment of thin section images, making sure that the section thickness is less than one half the nodule diameter, to avoid partial volume effects. In addition, such scans should be performed using helical technique, during a single breath hold, in order to avoid scan plane misregistration and motion artifact. It should, however, be remembered that not all calcifications seen at CT indicate benignancy. Benign forms of calcification include diffuse, near complete, laminated or central nidus patterns, and these lesions usually represent old granulomas. Hamartomas may contain coarse, scattered “popcornlike” calcification, either with or without foci of fat. (Depiction of fat within a nodule is essentially diagnostic for a pulmonary hamartoma, regardless of the presence or absence of calcification, and, like calcification, is best detected using thin sections.)
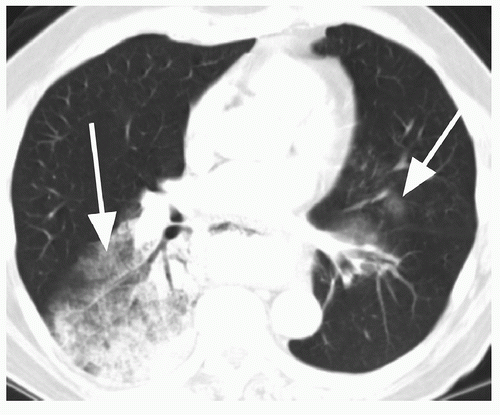
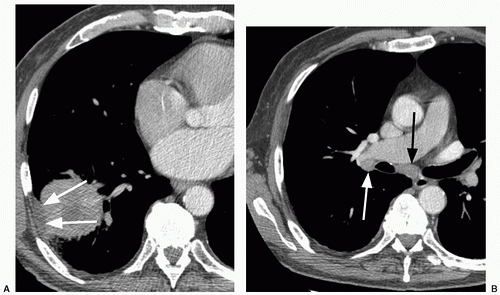
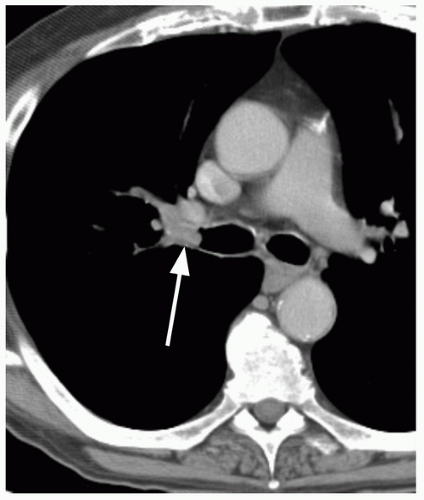
47 On the other hand, malignant forms of calcification include amorphous, stippled, or eccentric patterns (
Fig. 26.9). Approximately 10% of lung cancers
48 and up to 30% of carcinoids
49 contain calcification. Malignancies may calcify if dystrophic calcification is formed by the tumor cells, if the neoplasm arises in a preexisting, calcified scar, or if the tumor engulfs a nearby granuloma.
If morphologic features are indeterminate for benignancy, various diagnostic procedures may be attempted. One technique is the assessment of contrast enhancement. Preliminary studies with follow-up of noncalcified SPNs have reported that all or nearly all malignant SPNs enhance by at least 20 Hounsfield units (HU) within 2 to 4 minutes after contrast injection; few benign SPNs enhance to that degree.
50,
51,
52 Based on these promising preliminary results, a multicenter study
53 evaluated 356 SPNs that were ≥5 mm, solid, relatively spherical, homogeneous, and without calcification or fat on noncontrast images. Contrast-enhanced, single-or dual-detector helical CT images were obtained at 1, 2, 3, and 4 minutes after onset of injection (3-mm collimation, 420 mgI/kg, 300 mgI/mL administered at 2 mL/sec). Using a threshold increase in attenuation of 15 HU resulted in 98% sensitivity, 58% specificity, and 77% accuracy in diagnosing malignancy. Prevalence of malignancy in this patient group was 48% (171 of 356 nodules). A later study using a four-slice multidetector scanner and a slightly faster contrast injection rate found nearly identical results and also noted that peak attenuation of the nodules correlated with microvessel density.
54 Although uncommon, false-negative exams may occur occasionally in mucin-producing or necrotic tumors. Because of the latter pitfall, it is recommended that the technique not be used in large (>2 cm), potentially necrotic lesions. Given its overall high sensitivity, it is somewhat surprising that this technique has not become a standard tool in the workup of SPNs. However, another study demonstrated overlap in enhancement of malignant lesions and benign, active inflammatory lesions,
55 which may explain the low specificity and the current minor role of this technique.
Some preliminary work has been published on the use of combined contrast wash-in and wash-out, as an attempt to increase the specificity of contrast enhancement techniques. One
study examined 107 nodules (49 malignant, 58 benign) using multidetector helical CT images obtained out to 15 minutes after the onset of contrast injection.
56 Using an increase in attenuation (wash-in threshold) of ≥25 HU resulted in 100% sensitivity, 48% specificity, and 72% accuracy in diagnosing malignancy; however, the addition of a wash-out threshold of 5 to 31 HU led to greatly improved results, showing 94% sensitivity, 90% specificity, and 92% accuracy. False-positive cases were seen in pneumonias and false negatives in adenocarcinomas. Only 57% of malignancies reached peak enhancement within 2 minutes; 92% reached peak within 5 minutes and 98% within 9 minutes. These data suggest that images should be obtained out to at least 9 minutes after administration of the contrast bolus. These results are quite promising; however, a larger, follow-up study by the same group found somewhat less sanguine findings (89% sensitivity, 79% specificity, and 84% accuracy) and suggested that the combination of wash-in plus evaluation of morphologic features gives equivalent results to wash-in plus wash-out.
57The degree of lung nodule contrast enhancement has been used not only for diagnostic purposes, but also for regional staging. A recent report has suggested that peak enhancement of a lung nodule ≥110 HU or net enhancement ≥60 HU is indicative of the presence of mediastinal nodal metastases, showing sensitivity, specificity, and accuracy figures of 65%, 89%, and 83%, respectively; these figures were similar to the results of
18F-fluorodeoxyglucose-positron emission tomography (FDG-PET) scanning, performed in the same group of patients.
58Magnetic resonance imaging (MRI) is infrequently used for the detection and characterization of pulmonary nodules. The use of 2D half-Fourier single-shot turbo spin echo (HASTE) sequences allows the detection of pulmonary nodules greater than 5 mm in diameter. In a study using this sequence with MDCT as the gold standard, the sensitivity of MRI was 92% for lesions greater than 3 mm and 98% for nodules greater than 5 mm.
59 However, characterization using MRI is more difficult, partly because calcification is hard to identify on MRI. In an early study of 28 patients with SPNs, it was suggested that signal intensity measurements of nodules on dynamic contrast-enhanced MR studies may provide information about the nature of the nodules
60; in addition, other investigations have suggested that dynamic contrast enhanced MRI, including relative enhancement and rate of enhancement, may be helpful in differentiating benign from malignant nodules.
61 In a study by Schaefer et al.,
62 time-intensity curves showing contrast enhancement profiles were 100% sensitive and 75% specific for malignancy. The absence of enhancement and thin, peripheral rim enhancement are features suggesting a benign lesion.
61,
62,
63,
64In many centers, nodules that are suspicious for malignancy at CT are percutaneously biopsied using fluoroscopic or CT guidance; this is a relatively safe procedure and high accuracies have been reported, with positive predictive values (PPV) of at least 99%. The key number, however, is the negative predictive value (NPV): How reliable is a negative result? Can a nodule with a negative biopsy be safely watched, or should it be resected regardless of the biopsy result? Reported NPVs for fine-needle aspiration biopsy (FNAB) of lung nodules range from 59% to 82%.
65,
66,
67,
68 There is little data regarding the NPV of core needle biopsies in this setting, and the available results are also somewhat conflicting, ranging from 67% to 92%.
68,
69,
70,
71 The NPVs for core biopsies showing nonspecific benign tissue or insufficient tissue for diagnosis were 76% and 50%, respectively, according to one recent study; on the other hand, the NPV for a biopsy revealing a specific, benign diagnosis, such as hamartoma or fungus, approached 100%.
69 Published studies have suggested that the use of core needle biopsy technique increases the frequency of obtaining a specific benign diagnosis, compared to FNAB
72,
73; the proportion of specific benign diagnoses compared to all benign diagnoses ranged from 21% to 83% in recent investigations.
68,
69,
70,
71 Given these results, surgeons at many institutions believe that a percutaneous biopsy of an SPN is not indicated: except for the uncommon instance when a specific benign diagnosis is established, they will resect the nodule regardless of the biopsy results. (An exception might occur in the patient with a history of previous extrathoracic primary neoplasm.) At other institutions, where percutaneous biopsy is routinely performed for SPNs, it is advocated that a nonspecific negative biopsy be followed by a repeat biopsy. If the repeat biopsy is also negative for malignancy, then close follow-up is advised.
The various approaches to the SPN described previously have largely been overshadowed by the growing acceptance of PET scanning. PET has the obvious advantage of evaluating the metabolic behavior of the nodule rather than its morphology, and it is of great value in differentiating benign and malignant SPNs. PET is covered in
Chapter 27.
Whereas in the general population, the major issue with regard to an SPN is distinguishing the benign nodule from the malignant nodule, the issue is slightly different in the patient with a current or previous extrapulmonary primary cancer. In this type of patient, it can be important to distinguish between a solitary metastasis and a new bronchogenic carcinoma. The relative likelihood that a new SPN seen on a chest radiograph is a solitary metastasis versus a new lung cancer depends on the histology of the previous primary tumor. In some instances, the odds favor a new lung primary, such as for head and neck carcinoma (15.8:1), bladder carcinoma (8.3:1), and cervical carcinoma (6:1).
74 In fact, with some primaries, all malignant SPNs in one series were lung cancers (prostate, 26 patients; stomach, 7 patients; esophagus, 4 patients; pancreas, 3 patients). In other cases, a solitary metastasis is favored, such as in patients with soft tissue sarcoma (17.5:1), osteosarcoma (6.7:1), melanoma (4.1:1), and testicular carcinoma (2:1)
74 With most primaries the answer is in between, but slightly favoring lung cancer; examples include breast carcinoma (1.7:1), colon carcinoma (1.4:1), renal cell carcinoma (1.2:1), and endometrial carcinoma (1.1:1).
74Because CT is more sensitive at detecting lung nodules, the CT demonstration of a SPN more reliably indicates that there is really only one nodule. A recent study used CT to readdress the issue of a patient with a previous extrathoracic primary neoplasm and a new SPN.
75 In this study, breast cancer was
grouped with cancers of bladder, cervix, biliary tree, esophagus, ovary, prostate, and stomach, and the overall result was that 26 SPNs were lung cancers, and 8 were solitary metastases (3.3:1). For head and neck cancer, the ratio of lung cancers to solitary metastases was 8.3:1. For patients with carcinoma of the salivary glands, adrenal glands, colon, kidney, thyroid, thymus, or uterus the corresponding ratio was 0.8:1, whereas patients with melanoma, sarcoma, or testicular carcinoma had many more solitary metastases than new lung primaries (3.8:1).
75