Connective Tissue Diseases & the Heart: Introduction
The connective tissue diseases are immune-mediated inflammatory diseases, primarily of the musculoskeletal system; however, they frequently also involve the cardiovascular system. The most important of these diseases are systemic lupus erythematosus, rheumatoid arthritis, scleroderma, ankylosing spondylitis, polymyositis/dermatomyositis, and mixed connective tissue disease. They affect the valve leaflets, coronary arteries, pericardium, myocardium, conduction system, and great vessels with different rates of prevalence and degrees of severity. Although heart involvement in patients with connective tissue diseases contributes significantly to their morbidity and mortality rates, there is a large discrepancy between clinically recognized heart disease, echocardiography studies, and postmortem series. Furthermore, the pathogenesis, natural history, and effects of therapy are incompletely understood. Increased awareness and better understanding of the cardiovascular diseases associated with connective tissue diseases may lead to earlier recognition and treatment with consequent decreased morbidity and mortality. Finally and of importance, patients with connective tissue diseases and associated heart disease should be managed by both cardiologists and rheumatologists given the potential morbidity and mortality of their heart disease and the complexity and potential harm of their pharmacotherapy.
Systemic Lupus Erythematosus
- Musculoskeletal and mucocutaneous manifestations of systemic lupus erythematosus (SLE).
- Libman-Sacks vegetations, atrioventricular (AV) valve regurgitation, myocarditis, vascular thrombotic disease, and SLE.
- Cardioembolism and SLE.
- Acute pericarditis with antinuclear antibodies detected in the pericardial fluid.
Systemic lupus erythematosus (SLE) is a multisystem chronically recurrent inflammatory disease that affects the musculoskeletal, mucocutaneous, visceral, and central nervous systems. Symptoms include fatigue, myalgias, arthralgias or arthritis, photosensitivity, and serositis. The prevalence of SLE varies widely, from 4 to 250 cases per 100,000 persons. It is more frequent in a patient’s relatives than in the general population. SLE is predominantly seen in females, with a female-to-male ratio of 10:1. The pathophysiology of the disease is related to the multiorgan deposition of circulating antigen–antibody complexes and activation of the complement system, leading to humoral- and cellular-mediated inflammation.
Although SLE affects the cardiovascular system with varied frequency and degrees of severity, cardiovascular disease is the fourth most important cause of death in SLE patients (after infectious, renal, and central nervous system diseases) during the earlier course of disease, but later on, cardiovascular disease is a predominant cause of their death. The most significant SLE-associated heart diseases are valvular heart disease, arterial or venous thrombosis and systemic thromboembolism, coronary artery disease (CAD), and pericarditis. Myocarditis or cardiomyopathy and cardiac arrhythmias or conduction disturbances are less common.
Regarding the pathogenesis of SLE-associated cardiovascular disease, it is believed, as it is for the primary disease, that the immune complex deposition and complement activation lead to an acute, chronic, or recurrent inflammation of the valve leaflets, endocardium, vascular endothelium, pericardium, myocardium, or conduction system. The presence in these tissues of immune complexes, complement, antinuclear antibodies, lupus erythematosus cells, mononuclear inflammatory cells, necrosis, hematoxylin bodies, and deposits of fibrin and platelet thrombi support this theory. Many studies suggest that antiphospholipid antibodies (aPL) (immunoglobulin [Ig] A, IgG, or IgM anticardiolipin antibodies [aCL], lupus-anticoagulant [LA], or antibodies to plasma phospholipid-binding protein β2-glycoprotein I) cause cardiovascular injury. These antibodies, present in as many as half of SLE patients, are directed against negatively charged phospholipids present in the membrane of endothelial cells causing endothelial dysfunction, endocardial and/or vascular injury, and increased endocardial, arterial, and/or venous thrombogenesis.
Valvular heart disease is the clinically most important and frequent of the SLE-associated cardiovascular manifestations. Valvular heart disease is associated with an increased morbidity and mortality of SLE patients. It has been categorized as vegetations (Libman-Sacks endocarditis), leaflet thickening, valve regurgitation, and infrequently, valve stenosis. Although the true prevalence of clinically recognized valve disease is unknown, recent transesophageal echocardiography (TEE) series showed rates of at least 40%. Although not consistently demonstrated, rates of valve disease are probably higher in patients who have had SLE for more than 5 years, in those treated with corticosteroids, in those with higher disease damage scores, in those with moderate to high levels of aPL, and in those older than 50 years of age.
The pathogenesis of SLE valve disease includes (1) an immune complex–mediated inflammation with subendothelial deposition of immunoglobulins and complement leading to an increased expression of α3β1-integrin on the endothelial cells; (2) increased amount of collagen IV, laminin, and fibronectin; (3) proliferation of blood vessels; (4) inflammation and fibrosis; and finally, (5) commonly associated increased local or systemic thrombogenesis.
The proposed mechanisms of valve damage by aPL include (1) binding of aPL, which induces activation of endothelial cells and upregulation of the expression of adhesion molecules, secretion of cytokines, and abnormal metabolism of prostacyclins; (2) increased oxidized low-density lipoprotein (LDL) (LDL taken up by macrophages leads to macrophage activation and further damage to endothelial cells); (3) aPL interference with the regulatory functions of prothrombin and with the production of prostacyclin and endothelial relaxing factor, protein C, protein S, and tissue factor; and (4) a heparin-like–induced thrombocytopenia. All of these factors lead to increased vasoconstriction, platelet aggregation, and thrombus formation.
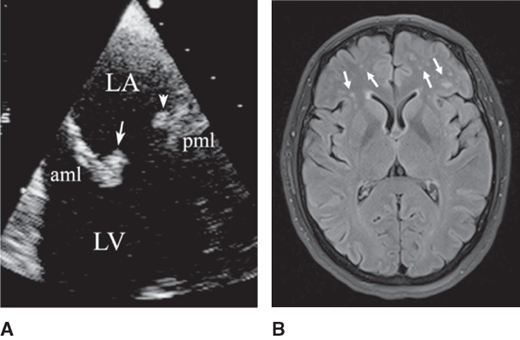
Considered pathognomonic of SLE-associated valve disease, noninfective valve vegetations are almost exclusively seen on the mitral and aortic valves. Most vegetations are located on the coaptation portions of the leaflets, the atrial side for the mitral valve, and the aortic vessel or ventricular side for the aortic valve. The valve vegetations are usually sessile, are less than 1 cm2 in size, have irregular borders and heterogeneous echodensity, and have no independent motion (Figures 35–1 and 35–2). Most valves with vegetations have associated thickening or regurgitation. Although valve vegetations have been seen more commonly in younger persons (< 40 years), their temporal association with SLE activity, severity, duration, and therapy has been variable.
Figure 35–1.
Recurrent strokes and mitral valve thickening with a large Libman-Sacks vegetation in a 47-year-old woman with systemic lupus erythematosus. A: This transesophageal echocardiographic (TEE) four-chamber view shows diffuse thickening predominantly of the mid and tip portions of the anterior (aml) and posterior (pml) mitral leaflets. A large vegetation with heterogeneous echoreflectance and irregular borders is noted on the atrial side of the posterior mitral leaflet (arrow). Moderate mitral regurgitation was present. LA, left atrium; LV, left ventricle; RV, right ventricle. B: This T2-weighted magnetic resonance imaging of the brain demonstrates generalized cortical atrophy, multiple areas of old cerebral infarcts characterized by loss of both gray and white matter (arrows) in a cortical and subcortical pattern. Also, multiple areas of deep white matter abnormality consistent with widespread ischemic cerebrovascular disease are noted.
Figure 35–2.
Mitral valve Libman-Sacks vegetations in a 37-year-old woman with systemic lupus erythematosus and a stroke. A: This close-up transesophageal echocardiographic view demonstrates a Libman-Sacks vegetation on the atrial side and tip of the anterior mitral leaflet (aml) (arrow) and a second vegetation on the midportion of the posterior leaflet (pml) (arrowhead). Associated diffuse thickening of both mitral leaflets is also noted. Moderate mitral regurgitation was demonstrated by color Doppler. LA, left atrium; LV, left ventricle. B: This fluid-attenuated inversion recovery magnetic resonance imaging of the brain demonstrates multiple and bilateral frontal subcortical white matter abnormalities (arrows) consistent with small cerebral infarcts.
Pathologic examination reveals that active Libman-Sacks vegetations have central fibrinoid necrosis with fibroblastic proliferation and fibrosis, surrounded by mononuclear and polymorphonuclear cellular infiltration, small hemorrhages, and platelet or fibrin thrombus. Healed vegetations have central fibrosis, minimal or no inflammatory cell deposition, and no or hyalinized and endothelialized thrombus. Active, healed, and mixed vegetations can be seen in the same valve.
Leaflet thickening with or without abnormal mobility results from replacement of the normal spongiosum and endothelial layers by postinflammatory fibrous tissue and infrequently by calcification. Valve thickening may be seen in up to half of patients; it is generally diffuse, with greater involvement of the middle and tip portions (see Figures 35–1 and 35–2). When leaflet thickening is localized, the basal, middle, and tip portions are equally affected. Valve thickening predominantly affects the mitral and aortic valves and is commonly associated with valve regurgitation, valve vegetations, or both. In young patients with no atherogenic risk factors, associated valve calcification is uncommon (< 5%). However, in middle-age patients with traditional atherogenic risk factors, mitral annular and aortic valve calcifications are common (20%).
This abnormality is predominantly mild in severity and therefore usually clinically silent. Although the prevalence of regurgitation is similar for the mitral, tricuspid, and pulmonic valves (about 50–75%) and the lowest for the aortic valve (25%), the mitral and aortic valves are those most commonly associated with complications. Mitral or aortic valve stenosis associated with respective valve regurgitation is uncommon.
Unless it is severe, valve disease is generally asymptomatic or overshadowed by the musculoskeletal and systemic inflammatory symptoms. However, subclinical valve disease is commonly first manifested with cardioembolism. Recent studies report that valve disease detected by TEE, especially mitral valve vegetations, are two to four times more common in patients with focal ischemic brain injury on magnetic resonance imaging (MRI), in those with stroke or transient ischemic attack (TIA), and in those with nonfocal neuropsychiatric manifestations of cognitive dysfunction, acute confusional state, seizures, or psychosis (see Figures 35–1 and 35–2). In these series, valve vegetations were strong independent predictors of brain injury and focal or nonfocal neuropsychiatric manifestations. These data suggest that valve disease in lupus patients is a source of fibrin or platelet macro- or microembolism to the brain. Also, severe valve regurgitation resulting from recurrent or acute native and bioprosthetic valvulitis, noninfective mitral valve chordal rupture, or infective endocarditis may occur in ≤ 4% of patients per year. Infective endocarditis can mimic, accompany, or trigger a flare of SLE and lead to severe valvular dysfunction, heart failure, and death from septicemia. Similarly, a flare of SLE can clinically and echocardiographically mimic infective endocarditis (pseudo-infective endocarditis). A low white count, elevated aPL and anti-DNA antibodies, depressed complements, and negative or low C-reactive protein support the diagnosis of active SLE with pseudo-infective endocarditis.
The physical findings of musculoskeletal and mucocutaneous disease generally predominate in SLE patients, even in those with cardiovascular disease. If moderate-to-severe mitral or aortic regurgitation or stenosis is present, the auscultatory findings found on physical examination will be typical. Less significant degrees of regurgitation (these are the majority) may not be clinically detected or may be mistaken for functional murmurs related to fever, anemia, hypertension, or volume overload. In patients with focal (stroke and TIAs) and nonfocal neuropsychiatric syndromes of seizures, confusional state, and cognitive dysfunction, cardioembolism from Libman-Sacks vegetations should be considered.
Results of electrocardiographic (ECG) studies are nonspecific. Left atrial abnormality and left ventricular (LV) hypertrophy can be seen in patients with chronic and severe aortic or mitral regurgitation.
Cardiomegaly with LV and left atrial enlargement may be seen in the presence of significant mitral or aortic regurgitation.
Transthoracic color-flow Doppler echocardiography (TTE) is the most commonly applied technique for the diagnosis of SLE-associated valve disease. This technique accurately determines the presence and severity of valve regurgitation or stenosis and abnormal leaflet thickening, but not of vegetations. The prevalence of Libman-Sacks vegetations by TTE is less than 10%. This technique will also detect associated increased wall thickness, chamber enlargement, ventricular diastolic or systolic dysfunction, and associated left atrial and pulmonary hypertension. TEE is superior to TTE in detecting and characterizing SLE-associated valve masses and leaflet thickening. TEE detects valve vegetations in up to 35% of patients. Considering TEE as the standard, TTE has a low sensitivity (63% overall, 11% for valve vegetations), low specificity (58%), low negative predictive value (40%), and a moderate positive predictive value (78%) for detection of Libman-Sacks endocarditis. By serial TEE, Libman-Sacks vegetations resolve, appear de novo, or change their morphology over time and may not be temporally related to SLE activity, severity, duration, or therapy. Therefore, TEE is indicated to exclude sources of cardioembolism in patients with a focal neurologic defect or in patients with a nonfocal neurologic deficit (moderate or worse cognitive dysfunction, seizures, acute confusional state, or psychosis) and cerebral infarcts on MRI, and in patients with suspected complicating infective endocarditis. Although there are limited data, three-dimensional TEE may further improve the diagnosis and characterization of Libman-Sacks endocarditis.
Currently, prospective data are limited regarding whether corticosteroids, disease-modifying antirheumatic drugs (DMARDs) such as antimalarials (hydroxychloroquine and chloroquine), immunosuppressive therapy (eg, methotrexate, azathioprine, cyclophosphamide, mycophenolate), biologic response modifiers (eg, rituximab, belimumab, intravenous immunoglobulin), and plasmapheresis are beneficial in treating acute SLE-associated valve disease. In general, acute valvulitis complicated with valve vegetations and/or moderate or worse valve regurgitation may be associated with active SLE disease, and therefore, treatment focuses on managing both active SLE disease and endocardial inflammation with anti-inflammatory (pulse corticosteroids) and immunosuppressive agents.
Long-term anticoagulation is beneficial in patients with Libman-Sacks vegetations and previous systemic embolism independently of aPL. The role of antiplatelets or anticoagulants in patients with vegetations but no clinical evidence of embolism has not been defined, but probably should be considered.
Diuretics, vasodilators, valve repair, or prosthetic valve replacement is indicated in severe symptomatic valve disease, including those cases complicated by infective endocarditis. The mortality rate associated with valve repair may be similar to the rate in those without SLE, but for valve replacement in SLE patients, it is twice that for patients without SLE.
Pericarditis, with or without effusion or pericardial thickening, is common in postmortem series. Also, about half of lupus patients suffer at least one episode of symptomatic pericarditis. Most episodes are acute and are frequently associated with active SLE and valvulitis, myocarditis, pleuritis, or nephritis. Cardiac tamponade or constrictive pericarditis rarely occurs (< 2%).
The diagnosis of pericarditis is based on clinical manifestations rather than on the echocardiogram, because an effusion or pericardial thickening is frequently absent. Symptomatic pericarditis is generally acute and uncomplicated and is most commonly seen during flare-ups of the disease. Asymptomatic pericardial disease may be present in some patients. It is manifested by incidentally detected small effusions in most cases and far less frequently by pericardial thickening found on echocardiography. Asymptomatic pericardial disease is generally seen in patients with stable disease that is either mildly active or in remission. Occasionally, acute pericarditis, cardiac tamponade, or both may be the initial manifestation of SLE. Chronic constrictive pericarditis is rare. Infectious pericarditis is rare but catastrophic and most commonly caused by Staphylococcus aureus. Finally, a pericardial effusion in SLE patients may also be secondary to severe uremia or nephrotic syndrome.
Because it is frequently symptomatic, acute pericarditis is the SLE-related cardiovascular disease most often detected clinically. It may present with fever, tachycardia, pleuritic chest pain, and, on auscultation, the presence of a pericardial rub. If a large effusion is present, decreased heart sounds, jugular venous distention, and pulsus paradoxus may be noted.
Pericarditis typically yields serofibrinous, fibrinous, or, rarely, serosanguineous exudative fluid containing low complement level and antinuclear antibodies. By immunofluorescence, the pericardium shows granular deposition of immunoglobulins and C3.
The ECG most frequently shows no abnormalities or nonspecific ST segment and T–wave changes. The characteristic diffuse ST segment elevation with upward concavity and PR segment depression of acute pericarditis are common. Low voltage or electrical alternans may also be seen if a large pericardial effusion is present.
The chest radiography is generally of little diagnostic value because most patients with acute pericarditis have no—or only small—pericardial effusions. If a large pericardial effusion is present, cardiomegaly with a characteristic water-bottle shape may be seen.
Since pericardial chest pain can be masked by musculoskeletal or pleural pain, echocardiography has complementary diagnostic value. Echocardiography may demonstrate small pericardial effusions or none. Small, asymptomatic pericardial effusions have also been found in up to 20% of SLE patients hospitalized with active disease. However, the absence of an effusion on echocardiography does not exclude a clinically suspected pericarditis. In cases of pericarditis with large pericardial effusion and clinically suspected cardiac tamponade, echocardiography may demonstrate right atrial or ventricular diastolic collapse and significant respiratory variability of the mitral or tricuspid Doppler inflows, indicating the need for therapeutic pericardiocentesis. Also, echocardiographically guided pericardiocentesis has been successfully performed in lupus patients with hemodynamically significant pericardial effusions. In addition, serial follow-up echocardiography after pericardiocentesis or after anti-inflammatory therapy is helpful to guide the need of future interventions. Echocardiography is less useful in detecting pericardial thickening or calcification in cases of suspected chronic pericardial constriction.
These techniques are preferred methods for assessing pericardial thickening when the echocardiogram suggests constriction.
Pericarditis is an indicator of serositis and of severely active SLE disease. Therefore, for severe pericarditis, immunosuppressive therapy with intravenous methylprednisolone or high-dose oral corticosteroids, followed by intravenous cyclophosphamide, plasmapheresis, rituximab, or oral mycophenolate, can be considered. For more indolent, recurrent, or chronic pericarditis, antimalarials (hydroxychloroquine or chloroquine), colchicine, methotrexate, mycophenolate, or intravenous belimumab can be used. The use of nonsteroidal anti-inflammatory drugs (NSAIDs) is frequently limited by associated renal disease, thrombocytopenia, or anticoagulation.
Pericardiocentesis should be performed when large effusions are unresponsive to medical therapy and when cardiac tamponade or complicating infectious pericarditis with effusion is suspected.
Pericardiectomy has been performed in isolated cases of SLE-associated chronic pericardial constriction.
Myocarditis can be seen in autopsy series in up to 80% of patients with SLE; by contrast, only 20% of cases can be clinically detected. Myocardial disease in SLE patients has four principal causes. First, a primary acute, chronic, or recurrent myocarditis is the most common. Primary myocarditis may be associated with acute pericarditis (myopericarditis). Primary myocarditis with LV diastolic and, rarely, global or regional systolic dysfunction occurs in at least 10% of patients. Rarely, acute myocarditis complicated with heart failure may be the initial manifestation of active SLE. An association of cellular antigen Ro (SS-A) and La (SS-B) antibodies and this type of myocarditis has been established, but their primary pathogenic role is still undefined. The second most common cause of myocardial diastolic and uncommonly systolic dysfunction results from endothelial dysfunction–mediated microvascular CAD. Small-vessel vasculitis or epicardial coronary arteritis is rare. The third cause is myocardial dysfunction resulting from severe mitral or aortic regurgitation. Finally, a rare and potentially reversible chloroquine sulfate–induced dilated or restrictive cardiomyopathy has been reported.
Acute myocarditis typically manifests with fever, tachycardia, chest pain, and, rarely, symptoms of heart failure, arrhythmias, or conduction disturbances. Acute myocarditis may clinically, electrocardiographically, and by laboratory mimic acute myocardial infarction. The myocarditis is generally mild and usually does not cause LV systolic dysfunction. However, up to one-third of young patients with active SLE have asymptomatic diastolic dysfunction. Occasionally, severe dilated cardiomyopathy is seen. Characteristic manifestations of an acute coronary syndrome will be present in those patients with myocardial dysfunction secondary to coronary arteritis, coronary atherosclerosis, small-vessel vasculitis, acute coronary thrombosis without underlying atherosclerosis, or coronary embolism from aortic or mitral valve noninfective vegetations.
If diastolic or systolic dysfunction is present, tachycardia, fourth and third heart sounds, pulmonary rales, and edema may be found.
Nonspecific ST segment and T-wave abnormalities and atrial or ventricular ectopic complexes are common. Rarely, atrial or ventricular tachyarrhythmias can be detected.
Cardiomegaly may be present if dilated cardiomyopathy has developed.
Generally, no abnormalities are detected in acute myocarditis. When the myocarditis is severe, diffuse or regional wall motion abnormalities may be observed. Doppler echocardiography series, including tissue Doppler, strain, and strain rate in asymptomatic young patients without systemic or pulmonary hypertension and normal LV systolic function, have demonstrated up to one-third of LV and right ventricular (RV) diastolic dysfunction, predominantly of impaired relaxation. Diastolic dysfunction occurs three to four times more frequently in patients with active SLE. In unselected patients, the prevalence of asymptomatic LV systolic dysfunction is low (≤ 3%). These abnormalities may be related to subclinical acute or recurrent myocarditis, microvascular CAD, or hypertension.
Either first-transit or gated-acquisition radionuclide angiography also can be used to assess ventricular systolic and diastolic dysfunction, wall motion abnormalities, and chamber enlargement. In up to one-third of SLE patients, this technique has shown an abnormal ventricular function response to exercise, as evidenced by a fall or subnormal rise in ejection fraction and the appearance of new or worsened wall motion abnormalities indicative of myocarditis or CAD. Reversible, fixed, or mixed myocardial perfusion defects can be seen in patients with normal epicardial coronary arteries indicative of active or past myocarditis or abnormal coronary flow reserve or microvascular disease.
This technique improves the detection of subclinical and clinical myocarditis. Patchy epicardial and intramyocardial delayed gadolinium enhancement is characteristic of postinflammatory myocardial fibrosis.
Tissue samples may demonstrate SLE-associated myocarditis or cardiomyopathy when a clinical or serologic diagnosis cannot be made.
Mild elevation of troponin I will be more common than elevation of creatine phosphokinase (CPK) and may occur in the absence of ECG or echocardiographic abnormalities.
As for pericarditis, myocarditis usually indicates severely active SLE disease, and therefore, in-patient intravenous pulse cyclophosphamide or oral mycophenolate in addition to intravenous methylprednisolone (1–2 mg/kg/day) can be considered. Once patients are considered stable, outpatient therapy with oral mycophenolate, antimalarials (hydroxychloroquine or chloroquine), methotrexate, azathioprine, or intravenous belimumab or rituximab is used and the corticosteroids are gradually tapered. This therapy is usually continued for 6–12 months, and corticosteroids are titrated down based on overall SLE and cardiac clinical response.
Symptomatic therapy with NSAIDs or other analgesics, bed rest, and ECG monitoring for detection of arrhythmias are indicated. If symptomatic dilated cardiomyopathy is present, standard therapy of diuretics, vasodilators, and digoxin therapy are used.
Deep venous thrombosis, pulmonary embolism, and peripheral or cerebral arterial thrombosis are common in SLE patients. Acute coronary thrombosis or thromboembolism in the absence of angiographic CAD has also been reported. Both arterial and venous thrombotic events have been associated with aPL. Patients with SLE are subject to intracardiac thrombosis and cerebral or systemic thromboembolism independently of or exacerbated by aPL. Current data support that SLE cerebrovascular disease is commonly associated and likely causally related to cardioembolism from Libman-Sacks endocarditis. In recent series, mitral or aortic valve vegetations were two to four times more common and strong independent predictors of focal ischemic brain injury on MRI; stroke or TIA; and nonfocal neurologic dysfunction, such as cognitive dysfunction, acute confusional state, or seizures (see Figures 35–1 and 35–2). In fact, microembolic events during transcranial Doppler echocardiography are common in patients with cerebral ischemic events.
Although acute pleuritic chest pain and tachycardia could be related to the presence of pericarditis, pleuritis, or pneumonitis, they should prompt the suspicion of pulmonary embolism and deep vein thrombosis (DVT). Focal and nonfocal transient or permanent neurologic deficits are commonly due to cardioembolism from valvular disease and rarely due to vasculitis or cerebritis or atherosclerosis.
Antiphospholipid antibodies are highly associated with venous or arterial thrombotic events. However, these antibodies can be present in SLE patients without thrombosis and infrequently in patients who do not have SLE. Therefore, routine measurement of aPL to identify patients at high thrombotic risk and as a basis for prophylactic antiplatelet or anticoagulant therapy is still undefined.
TEE should be considered in SLE patients with focal neurologic deficits, in those with nonfocal neurologic deficits and focal brain injury on brain MRI, and in those with peripheral arterial thrombosis to exclude cardioembolism from Libman-Sacks vegetations.
These imaging methods of the lower extremities should be performed if DVT is suspected.
These methods should be considered if pulmonary embolism is clinically suspected.
Corticosteroids or immunosuppressive agents may be beneficial in patients with active SLE and noninfective vegetations with or without thrombosis or thromboembolism.
Anticoagulation with warfarin is the therapy of choice in patients with DVT, pulmonary embolism, and noninfective valve vegetations and stroke or TIA or suspected embolic cerebral infarcts on MRI.
Postmortem studies in SLE patients have demonstrated at least a 25% prevalence of CAD, but clinically evident disease or arteritis is probably less common. Clinical and subclinical functional or small-vessel CAD is more frequent than epicardial coronary disease in clinical series.
After controlling for traditional risk factors for CAD, the risk of functional (abnormal coronary vasodilation or microvascular disease) or subclinical atherosclerotic epicardial CAD in the form of coronary artery calcifications in lupus patients is three to eight times higher than matched controls. This form of subclinical CAD is predictive of a higher risk for future cardiovascular events (myocardial infarction [MI], stroke, death). Risk factors for CAD in SLE patients are a longer mean duration of the disease, a longer mean duration and dose of prednisone therapy, a high disease activity and damage score, lupus nephritis, and SLE–induced dyslipidemia (high levels of LDL phenotype B [the atherogenic phenotype], low levels of high-density lipoprotein [HDL], high levels of proinflammatory HDL, and high levels of triglycerides). Although, a high Framingham risk score is also an important predictor, this score by itself underestimates these patients’ cardiovascular risk.
The proposed pathogenetic mechanisms for CAD include activation of cellular and humoral immunity (including aPL) with activation of macrophages, CD4+CD28− T cells, and dendritic cells. The cytotoxicity of these cells to the endothelium and vascular wall results in decreased production of prostacyclin and prostaglandin I and consequently in increased vasoconstriction. Also, vascular wall cytotoxicity results in an increased thrombosis via release of platelet-derived growth factor and thromboxane A2. Cytotoxic cells also produce interferon-γ, which destabilizes atherosclerotic plaques by suppressing synthesis of collagen, increased proliferation of smooth muscle cells, and activation of macrophages to release free radicals and matrix metalloproteinases. Other proposed pathogenetic mechanisms for CAD include increased oxidation of LDL and increased production of inflammatory cytokines and chemokines such as heat shock proteins, C-reactive protein, rheumatoid factor, tumor necrosis factor-α, and interleukins. These cytokines are expressed on the endothelium of coronary arteries, recruit inflammatory cells, promote abnormal vascular smooth cell proliferation, and induce oxidative stress, endothelial apoptosis, and further upregulation of adhesion molecules and chemokines. A final proposed pathogenetic mechanisms for CAD is exacerbation of dyslipidemia (high levels of very–low–density lipoproteins and triglycerides and low levels of HDL), homocysteinemia, and insulin resistance. Uncommon pathogenetic factors include coronary arteritis, in situ coronary thrombosis, or embolization from a Libman-Sacks vegetation.
The presentation of CAD in SLE patients is not unique and involves stable or exertional angina, unstable angina, acute ST or non-ST elevation MI, or, rarely, heart failure from ischemic LV dysfunction. In addition, fatal MI can occur in SLE patients, and some data suggest an increased risk of myocardial rupture after MI in SLE patients treated with corticosteroids. Coronary arteritis should be suspected in a young patient with an acute ischemic syndrome, active SLE, and evidence of vasculitis affecting other organs. Also, coronary embolism or in situ thrombosis warrants consideration when MI occurs in the presence of a cardioembolic substrate (valve vegetations) or moderate to high levels of aPL.
Electrocardiography, exercise testing with or without perfusion scanning, and echocardiography can be used in SLE patients in whom CAD is suspected. However, the diagnostic value of these techniques is inferior to that in the general population due to their young age, female predominance, and lower prevalence of obstructive epicardial CAD. Electron-beam computed tomography (CT) has demonstrated a high prevalence (30–45%) of coronary calcification in asymptomatic patients. Cardiac MRI can detect subendocardial ischemia and small patchy areas of delayed contrast enhancement at rest and during exercise in up to 40% of patients with proven microvascular, but no epicardial, CAD on coronary angiography. A similarly high prevalence of perfusion abnormalities with normal coronary arteries has been reported using radioisotope myocardial perfusion imaging. Recent series in young SLE patients have also shown the development of premature carotid and aortic stiffness associated with intima media thickening and plaques, which may be markers of underlying premature epicardial or microvascular CAD. None of these techniques, including angiography, can reliably differentiate coronary arteritis from common coronary atherosclerosis. Suspected epicardial CAD may warrant coronary angiography because of the confounding clinical, echocardiographic, and myocardial perfusion features of functional or small-vessel CAD or lupus myocarditis.
If coronary arteritis is suspected, in-patient intravenous cyclophosphamide or oral mycophenolate and high-dose corticosteroids (1–2 mg/kg/day) may be considered initially and continued on out-patient basis after patient clinical stabilization. The duration of high-dose therapy is usually for several weeks and tailored based on overall SLE and cardiac clinical response, but in these cases, long-term immunosuppression and antimalarial therapy are necessary to prevent recurrent disease. Corticosteroids may have some additional danger for myocardial rupture in patients with recent transmural MI.
Except for the use of immunosuppressive agents in suspected arteritis, the medical therapy of acute and chronic epicardial CAD is not different from that in the general population. Both percutaneous coronary intervention (PCI) and bypass surgery have been successfully performed in SLE patients.
The prevalence of these abnormalities, including prolongation of the QT interval, is unknown. Although they are common with myocarditis and highly associated with the presence of anti-Ro/SS–A antibodies, no primary pathogenetic role of these antibodies has been demonstrated. Atrial fibrillation or flutter may be seen during episodes of acute pericarditis, myocarditis, pulmonary embolism, or pulmonary hypertension. Chronic conduction disturbances may be due to the inflammation and fibrosis of the conduction system frequently found at autopsy. ECG is the most valuable technique for detecting arrhythmias and conduction disturbances.
Although experience is limited, acute high-degree atrioventricular (AV) blocks more commonly seen in association with acute myocarditis or myopericarditis may resolve with the use of intravenous immunosuppressives and high-dose corticosteroids, and these drugs should be used before considering permanent pacing.
Temporary pacing is an alternative treatment for acute AV blocks. Permanent pacemakers should be used in cases of symptomatic high-grade AV blocks that are unresponsive to immunosuppressives and corticosteroids.
The overall survival rate of SLE patients is about 75% over 10 years. If the heart, lung, kidney, or central nervous system is clinically involved, the prognosis is worse. Cardiovascular disease is the third major cause of mortality in SLE patients, after infectious and renal diseases early in the course of disease, but it becomes a predominant cause, especially CAD, at the later stage. Valvular disease, myocardial disease, and CAD are all known to decrease the survival of SLE patients.
Rheumatoid Arthritis
- Clinical evidence of rheumatoid arthritis.
- Granulomatous valve disease, predominantly of the mitral and aortic valves.
- Pericarditis and myocarditis with granuloma on biopsy.
Rheumatoid arthritis is an immune-mediated chronic inflammatory disease characterized by morning stiffness, arthralgias, or arthritis, predominantly of the metacarpophalangeal or proximal interphalangeal joints; rheumatoid nodules; serum IgM or IgG rheumatoid factor and anticyclic citrullinated peptide (anti-CCP) antibody; and articular erosions seen on a radiograph. The disease prevalence is about 1%, and it affects females more than males with a ratio of 2–4:1. The natural history of the disease is such that the median life expectancy is reduced by 7 years in men and 3 years in women. The most common causes of death are articular and extra-articular complications such as atlantoaxial subluxation, cricoarytenoid synovitis, sepsis, heart disease, cardiopulmonary complications, and diffuse vasculitis. Rheumatoid arthritis patients with the worst prognosis are those with positive rheumatoid factor, nodular disease, and male gender.
Rheumatoid cardiovascular disease is produced by a nonspecific immune complex–mediated inflammation, vasculitis, or granulomatous deposition on the pericardium, myocardium, heart valves, coronary arteries, aorta, or the conduction system. Clinically apparent rheumatoid heart disease occurs in one-third of patients, compared with up to 80% in autopsy series. Rheumatoid heart disease may appear as pericarditis, myocarditis, valvular heart disease, atherosclerotic CAD, conduction disturbances, coronary arteritis, aortitis, or cor pulmonale. The sole presence of rheumatoid arthritis is now considered a primary pathogenic factor for premature development of CAD with also a threefold increase in cardiovascular events.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree